Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0668: Differential Expression of Interferon Related Genes in SLE Patients of Asian and European Ancestries Abstract
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- IR
Ian Rector, BA
AMPEL Biosolutions
Charlottesville, VA, United States
Abstract Poster Presenter(s)
Ian Rector1, Prathyusha Bachali2, Jinoos Yazdany3, Maria Dall'Era4, Amrie Grammer5 and Peter lipsky1, 1AMPEL Biosolutions, Charlottesville, VA, 2AMPEL BioSolutions, Redmond, WA, 3UCSF, San Francisco, CA, 4University of California, Division of Rheumatology, San Francisco, CA, 5AMPEL LLC, Charlottesville, VA
Background/Purpose: Interferon has been shown to play a role in the pathogenesis of SLE, but insufficient studies have been conducted into the differences in interferon response between patients of different ancestries. African ancestry patients have been shown to have greater interferon response than European ancestry (EA) patients related to the involvement of autoantibodies, but the differences between Asian ancestry (AsA) patients and EA patients are not well studied. This question was explored using a bulk RNA-Seq analysis from the CLUES (California Lupus Epidemiology Study) of both AsA and EA patients in four cell types: CD14 monocytes, CD19 B cells, CD4 T cells and NK cells.
Methods: We employed GSVA, or Gene Set Variability Analysis, a statistical method that focuses on enrichment of sets of genes rather than individual genes to examine the differences in IFN expression between the two ancestral groups. We initially used seven different interferon response signatures (IGS) encompassing the response to all Type 1 IFN (IFN Core), IFNA2, IFNB1, IFNW1 and IFNG as well as IL12 and TNF as controls. The Singscore R package was used to rank the genes of each of the seven IFN modules by expression, and create a more concise version of the IFN Core signature and the IFNA2 signature.
Results: AsA patients exhibited higher levels of IFN core and IFNA2 IGS than EA patients in all four cell types. GSVA was used to examine how the presence of different autoantibodies (anti-DNA, anti-RNP, anti-SSA, anti-Sm) was associated with the IGS. Patients who had autoantibodies tended to have higher IFN signatures, but not IL12 and TNF signatures, compared to patients who lacked autoantibodies. EA patients who lacked autoantibodies were generally IGS negative. In both AsA and EA SLE patients the IGS was significantly greater in subjects with anti-RNP autoantibodies.
Conclusion: These results show that AsA SLE patients have higher IGS than EA patients and this is manifest in all cell types. Moreover, the presence of the IGS is greatest is patients with anti-RNP autoantibodies, and especially in SLE patients of AsA. These results link the presence of the IGS to anti-RNP autoantibodies in AsA patients with SLE.
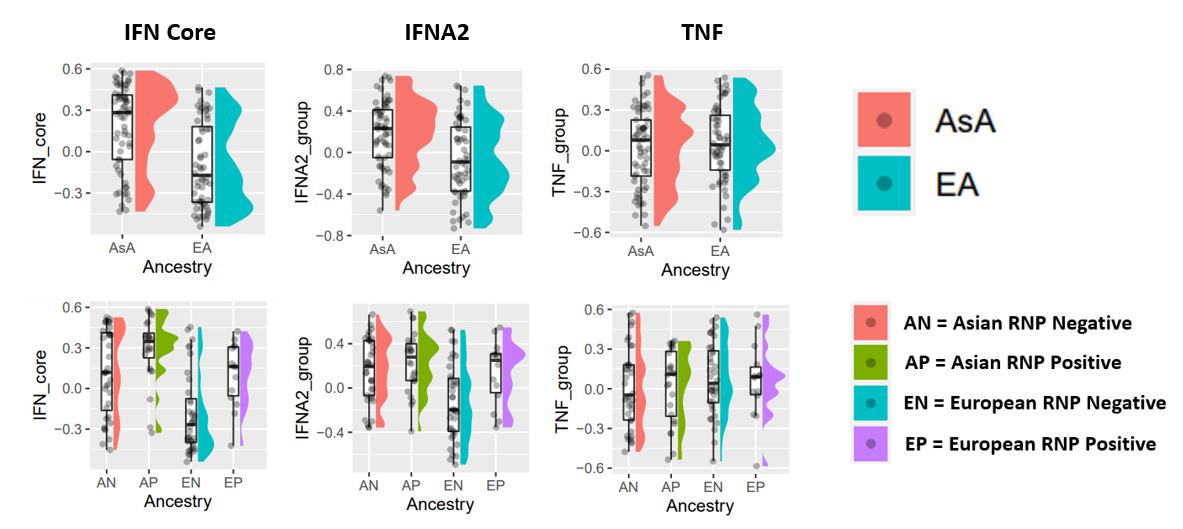 Figure 1: The IGS is more frequent in SLE patients of Asian ancestry (top) and in those with anti-RNP autoantibodies (bottom). Both plots are for the monocyte cell population.
Figure 1: The IGS is more frequent in SLE patients of Asian ancestry (top) and in those with anti-RNP autoantibodies (bottom). Both plots are for the monocyte cell population.
Disclosures: I. Rector, None; P. Bachali, None; J. Yazdany, AstraZeneca, Gilead, Bristol-Myers Squibb(BMS), Aurinia, Astra Zeneca, Pfizer; M. Dall'Era, GlaxoSmithKline (GSK), AstraZeneca, Aurinia, BMS, Amgen; A. Grammer, None; P. lipsky, None.
Background/Purpose: Interferon has been shown to play a role in the pathogenesis of SLE, but insufficient studies have been conducted into the differences in interferon response between patients of different ancestries. African ancestry patients have been shown to have greater interferon response than European ancestry (EA) patients related to the involvement of autoantibodies, but the differences between Asian ancestry (AsA) patients and EA patients are not well studied. This question was explored using a bulk RNA-Seq analysis from the CLUES (California Lupus Epidemiology Study) of both AsA and EA patients in four cell types: CD14 monocytes, CD19 B cells, CD4 T cells and NK cells.
Methods: We employed GSVA, or Gene Set Variability Analysis, a statistical method that focuses on enrichment of sets of genes rather than individual genes to examine the differences in IFN expression between the two ancestral groups. We initially used seven different interferon response signatures (IGS) encompassing the response to all Type 1 IFN (IFN Core), IFNA2, IFNB1, IFNW1 and IFNG as well as IL12 and TNF as controls. The Singscore R package was used to rank the genes of each of the seven IFN modules by expression, and create a more concise version of the IFN Core signature and the IFNA2 signature.
Results: AsA patients exhibited higher levels of IFN core and IFNA2 IGS than EA patients in all four cell types. GSVA was used to examine how the presence of different autoantibodies (anti-DNA, anti-RNP, anti-SSA, anti-Sm) was associated with the IGS. Patients who had autoantibodies tended to have higher IFN signatures, but not IL12 and TNF signatures, compared to patients who lacked autoantibodies. EA patients who lacked autoantibodies were generally IGS negative. In both AsA and EA SLE patients the IGS was significantly greater in subjects with anti-RNP autoantibodies.
Conclusion: These results show that AsA SLE patients have higher IGS than EA patients and this is manifest in all cell types. Moreover, the presence of the IGS is greatest is patients with anti-RNP autoantibodies, and especially in SLE patients of AsA. These results link the presence of the IGS to anti-RNP autoantibodies in AsA patients with SLE.
 Figure 1: The IGS is more frequent in SLE patients of Asian ancestry (top) and in those with anti-RNP autoantibodies (bottom). Both plots are for the monocyte cell population.
Figure 1: The IGS is more frequent in SLE patients of Asian ancestry (top) and in those with anti-RNP autoantibodies (bottom). Both plots are for the monocyte cell population.Disclosures: I. Rector, None; P. Bachali, None; J. Yazdany, AstraZeneca, Gilead, Bristol-Myers Squibb(BMS), Aurinia, Astra Zeneca, Pfizer; M. Dall'Era, GlaxoSmithKline (GSK), AstraZeneca, Aurinia, BMS, Amgen; A. Grammer, None; P. lipsky, None.

