Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0589–0628) RA – Etiology and Pathogenesis Poster
0625: Distinct Effects of JAK Inhibitor and TNF Inhibitors on Circulating B Cell Phenotypes in Rheumatoid Arthritis Patients
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- ME
Mehreen Elahee, MD
University of Pittsburgh Medical Center
Pittsburgh, PA, United States
Abstract Poster Presenter(s)
Mehreen Elahee1, Kathryne Marks1, Ifeoluwakiisi Adejoorin1, Lin Chen1, Derrick Todd1, Jonathan Coblyn1, Elena Massarotti1, Susan Ritter1, Daniel Solomon1, Michael Weinblatt2 and Deepak Rao1, 1Brigham and Women's Hospital, Boston, MA, 2Brigham and Women's Hospital, Harvard Medical School, Boston, MA
Background/Purpose: Multiple DMARDs are used to treat rheumatoid arthritis (RA) yet, there are currently few tools to assess if DMARD treatment influences pathologic immune cell activation. B cells play critical roles in the pathogenesis of RA and are an important therapeutic target. We have evaluated effects of two RA therapies, TNF inhibitors and JAK inhibitors, on circulating B cells. Defining the B cell phenotypes altered by either drug class may help to identify B specific cell populations associated with treatment response and provide biomarkers to assess effects of treatment on the active immune response.
Methods: Blood samples were collected from RA patients before and after treatment with JAK inhibitor (n=27) or TNF inhibitor (n=32). RA patients met the 2010 ACR-EULAR RA classification criteria. B cell subsets were analyzed using flow cytometry with the following markers: CD14, CD19, CD20, IgD, CD27, CD38, CD21, IgG, IgM, IgA, CD24 and CD11c. Clinical Disease Activity Index (CDAI) and electronic medical records review before and after treatment was used to categorize patients into treatment responders or non-responders. IgD and CD27 was used to identify the main B cell subsets: naïve (IgD+CD27-), switched memory (IgD-CD27+), non-switched memory (IgD+CD27+) and double negative (DN, IgD-CD27-), Plasmablasts (CD38++CD27+). The Naïve B cell gate was used to further quantify activated naïve B cells (aNAV, CD11c+ CD21-) and transitional B cells (CD38+CD24+). Wilcoxon paired tests were used for comparison between the two timepoints with Bonferroni correction.
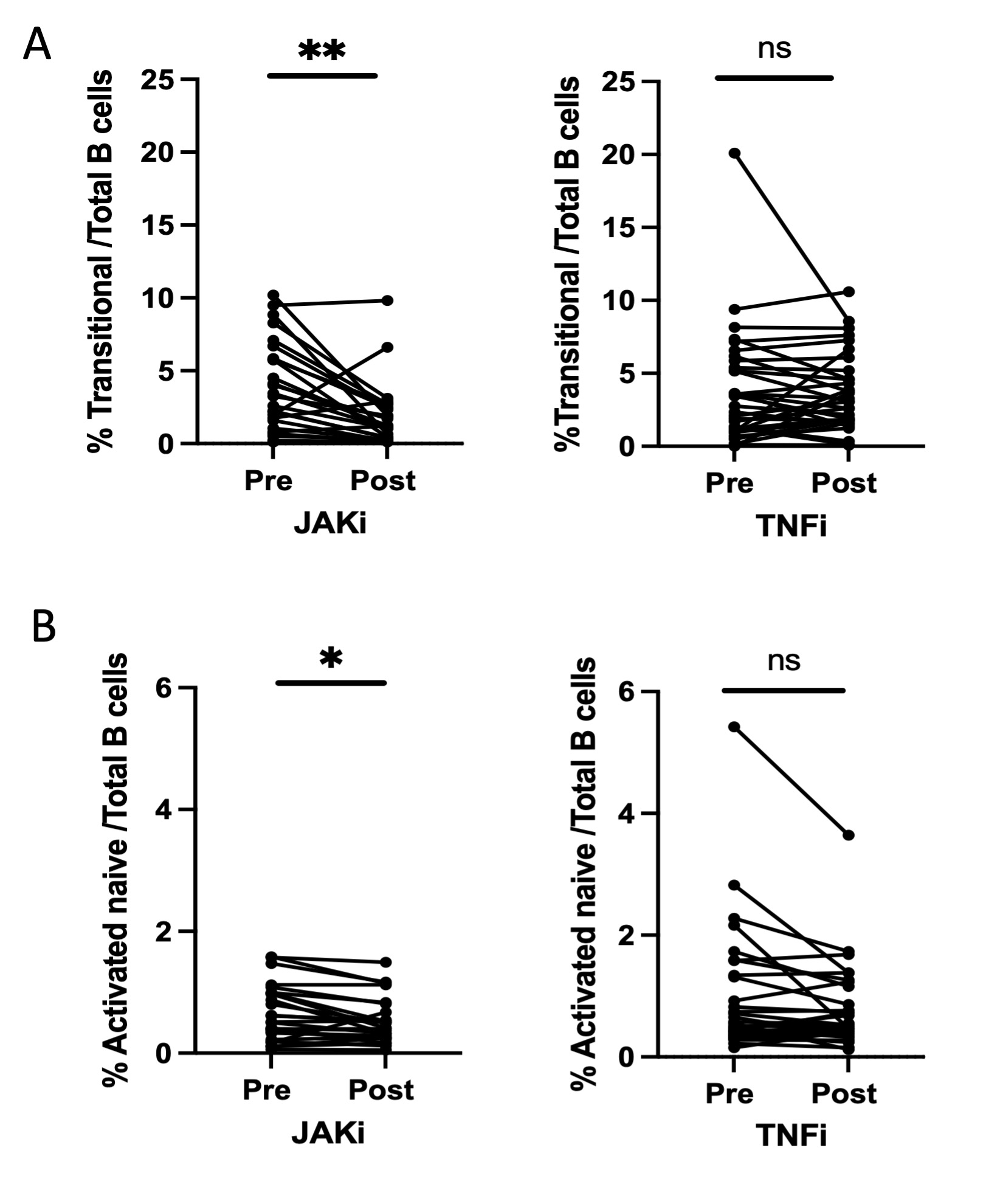
Results: Patients in both treatment groups had similar circulating levels of all B cell subsets at baseline. Comparison of the pre- and post-treatment frequencies of B cell subsets demonstrated that the proportion of transitional B cells decreased significantly after treatment with JAK inhibitor (p=0.002) but not with anti-TNF (p=0.94) (Fig 1A). Proportion of aNAV B cells also showed a significant decrease following treatment with JAK inhibitors (p=0.02) but not TNF inhibitors (p=0.17) (Fig 1B). The proportions of naïve B cells and plasmablasts did not change post treatment with JAK inhibitor (p=.68) or anti-TNF (p=.32). 19 out of 27 patients in the JAK inhibitor arm and 20 out of 32 patients in the TNF inhibitor arm were classified as treatment responders. Frequencies of B cell populations at the pre-treatment timepoint did not differ between responders and non-responders in either treatment arm. Among JAK inhibitor treated patients, both transitional B cells and aNAV B cells decreased comparably in treatment responders and non-responders. Decreases in these B cell populations were also comparable between seropositive (n=34) and seronegative (n=25) patients.
Conclusion: We found significant reductions in the transitional B cell and aNAV B cell populations in RA patients treated with JAK inhibitors, but not in patients treated with TNF inhibitors. Further understanding of the cellular changes associated with specific DMARDs may provide insights into the relevant mechanisms of action of different treatments and may provide biomarkers that can be quantified.
Disclosures: M. Elahee, None; K. Marks, None; I. Adejoorin, None; L. Chen, None; D. Todd, None; J. Coblyn, None; E. Massarotti, None; S. Ritter, Pfizer; D. Solomon, Amgen, AbbVie/Abbott, CorEvitas, Moderna, Janssen; M. Weinblatt, Eli Lilly, AbbVie/Abbott, aclaris, Amgen, Bristol-Myers Squibb(BMS), corevitas, Eqrx, genosco, GlaxoSmithKlein(GSK), Gilead, horizon therapeutics, johnson and johnson, scipher, canfite, inmedix; D. Rao, Janssen, Merck, Bristol-Myers Squibb, Scipher Medicine, HiFiBio, Inc., AstraZeneca, Pfizer.
Background/Purpose: Multiple DMARDs are used to treat rheumatoid arthritis (RA) yet, there are currently few tools to assess if DMARD treatment influences pathologic immune cell activation. B cells play critical roles in the pathogenesis of RA and are an important therapeutic target. We have evaluated effects of two RA therapies, TNF inhibitors and JAK inhibitors, on circulating B cells. Defining the B cell phenotypes altered by either drug class may help to identify B specific cell populations associated with treatment response and provide biomarkers to assess effects of treatment on the active immune response.
Methods: Blood samples were collected from RA patients before and after treatment with JAK inhibitor (n=27) or TNF inhibitor (n=32). RA patients met the 2010 ACR-EULAR RA classification criteria. B cell subsets were analyzed using flow cytometry with the following markers: CD14, CD19, CD20, IgD, CD27, CD38, CD21, IgG, IgM, IgA, CD24 and CD11c. Clinical Disease Activity Index (CDAI) and electronic medical records review before and after treatment was used to categorize patients into treatment responders or non-responders. IgD and CD27 was used to identify the main B cell subsets: naïve (IgD+CD27-), switched memory (IgD-CD27+), non-switched memory (IgD+CD27+) and double negative (DN, IgD-CD27-), Plasmablasts (CD38++CD27+). The Naïve B cell gate was used to further quantify activated naïve B cells (aNAV, CD11c+ CD21-) and transitional B cells (CD38+CD24+). Wilcoxon paired tests were used for comparison between the two timepoints with Bonferroni correction.
Results: Patients in both treatment groups had similar circulating levels of all B cell subsets at baseline. Comparison of the pre- and post-treatment frequencies of B cell subsets demonstrated that the proportion of transitional B cells decreased significantly after treatment with JAK inhibitor (p=0.002) but not with anti-TNF (p=0.94) (Fig 1A). Proportion of aNAV B cells also showed a significant decrease following treatment with JAK inhibitors (p=0.02) but not TNF inhibitors (p=0.17) (Fig 1B). The proportions of naïve B cells and plasmablasts did not change post treatment with JAK inhibitor (p=.68) or anti-TNF (p=.32). 19 out of 27 patients in the JAK inhibitor arm and 20 out of 32 patients in the TNF inhibitor arm were classified as treatment responders. Frequencies of B cell populations at the pre-treatment timepoint did not differ between responders and non-responders in either treatment arm. Among JAK inhibitor treated patients, both transitional B cells and aNAV B cells decreased comparably in treatment responders and non-responders. Decreases in these B cell populations were also comparable between seropositive (n=34) and seronegative (n=25) patients.
Conclusion: We found significant reductions in the transitional B cell and aNAV B cell populations in RA patients treated with JAK inhibitors, but not in patients treated with TNF inhibitors. Further understanding of the cellular changes associated with specific DMARDs may provide insights into the relevant mechanisms of action of different treatments and may provide biomarkers that can be quantified.

Disclosures: M. Elahee, None; K. Marks, None; I. Adejoorin, None; L. Chen, None; D. Todd, None; J. Coblyn, None; E. Massarotti, None; S. Ritter, Pfizer; D. Solomon, Amgen, AbbVie/Abbott, CorEvitas, Moderna, Janssen; M. Weinblatt, Eli Lilly, AbbVie/Abbott, aclaris, Amgen, Bristol-Myers Squibb(BMS), corevitas, Eqrx, genosco, GlaxoSmithKlein(GSK), Gilead, horizon therapeutics, johnson and johnson, scipher, canfite, inmedix; D. Rao, Janssen, Merck, Bristol-Myers Squibb, Scipher Medicine, HiFiBio, Inc., AstraZeneca, Pfizer.

