Back
Poster Session B
Antiphospholipid Syndrome
Session: (0671–0694) Antiphospholipid Syndrome Poster
0687: DNA- and NET-Binding Beta-2 Glycoprotein I in a Large Cohort of Antiphospholipid Antibody-Positive Patients
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- KK
Katarina Kmetova, BSc, MSc
Division of Rheumatology, University of Michigan, Ann Arbor, Michigan, USA
Ann Arbor, MI, United States
Abstract Poster Presenter(s)
Katarina Kmetova1, Srilakshmi Yalavarthi2, Claire Hoy2, Cyrus Sarosh3, Noah Peters2, Tristin Smith2, Sherwin Navaz2, Yu Zuo2 and Jason S Knight4, 1Division of Rheumatology, University of Michigan, Ann Arbor, MI, 2University of Michigan, Ann Arbor, MI, 3University of Michigan, Temperance, MI, 4University of Michigan, Division of Rheumatology, Ann Arbor, MI
Background/Purpose: Beta-2 glycoprotein I (β2GPI) is an abundant plasma protein and a critically important autoantigen in APS. While β2GPI has been reported to have antioxidant, anticoagulant, and scavenging functions, its most important physiological roles continue to be debated. Old but intriguing reports have suggested that β2GPI may function as a DNA-binding protein. Here, we aimed to dissect the interaction between β2GPI and not only DNA, but also neutrophil extracellular traps (NETs). NETs are prothrombotic tangles of nuclear DNA, histones, and granular proteins extruded from activated neutrophils in APS. We also sought to evaluate for the presence and potential clinical significance of β2GPI-DNA complexes in a large cohort of antiphospholipid antibody (aPL)-positive patients.
Methods: We used an electrophoretic mobility shift assay (EMSA) to assess potential direct interaction between β2GPI and either genomic DNA or purified NETs. Such interactions were also assessed by immunofluorescence microscopy after inducing NET formation with phorbol 12-myristate 13-acetate (PMA); this was done in the presence of human serum as a source of β2GPI. Finally, we developed a novel sandwich ELISA to measure circulating β2GPI-DNA complexes in the plasma of patients.
Results: By EMSA, β2GPI caused a dose-dependent retardation of the mobility of both genomic DNA and isolated NETs through an agarose gel (Fig 1A-B). By immunofluorescence microscopy, we observed β2GPI in intimate association with NET strands (Fig 1C). We next evaluated the presence of circulating β2GPI-DNA complexes in the plasma of aPL-positive patients (78% meeting criteria for APS, Fig 2A). As compared with healthy controls, many aPL-positive patients demonstrated elevated levels of β2GPI-DNA complexes (p< 0.01, Fig 2B). No difference was found between patients who met classification criteria for APS and those who did not (p=0.10, Fig 2C). There was also no difference in circulating β2GPI-DNA complexes in the presence or absence of SLE (Fig 2D). Notably, β2GPI-DNA complexes demonstrated a strong positive correlation with neutrophil elastase-DNA complexes (r=0.50, p< 0.001), an established biomarker for NETs. There was a negative correlation with absolute neutrophil count (r=-0.26, p=0.007), suggesting a potential association with neutrophil cell death. We also considered whether β2GPI-DNA correlated with levels of aPL. While no such correlation was appreciated for traditional aPL tests such as anticardiolipin antibodies, anti-β2GPI antibodies, and lupus anticoagulant, we did find a positive correlation with anti-PS/PT IgG (r=0.18, p< 0.05). There was also a non-significant association with anti-dsDNA antibodies (r=0.20, p=0.053).
Conclusion: In summary, our data confirm the direct association of β2GPI with DNA and NETs. Many aPL-positive patients have high levels of circulating β2GPI-DNA complexes, which likely relate to the heightened neutrophil activation and NET formation that are integral to aPL-mediated thromboinflammation. The extent to which the β2GPI-NET interaction may amplify autoantigen spread and perpetuate autoimmunity in APS warrants further investigation.
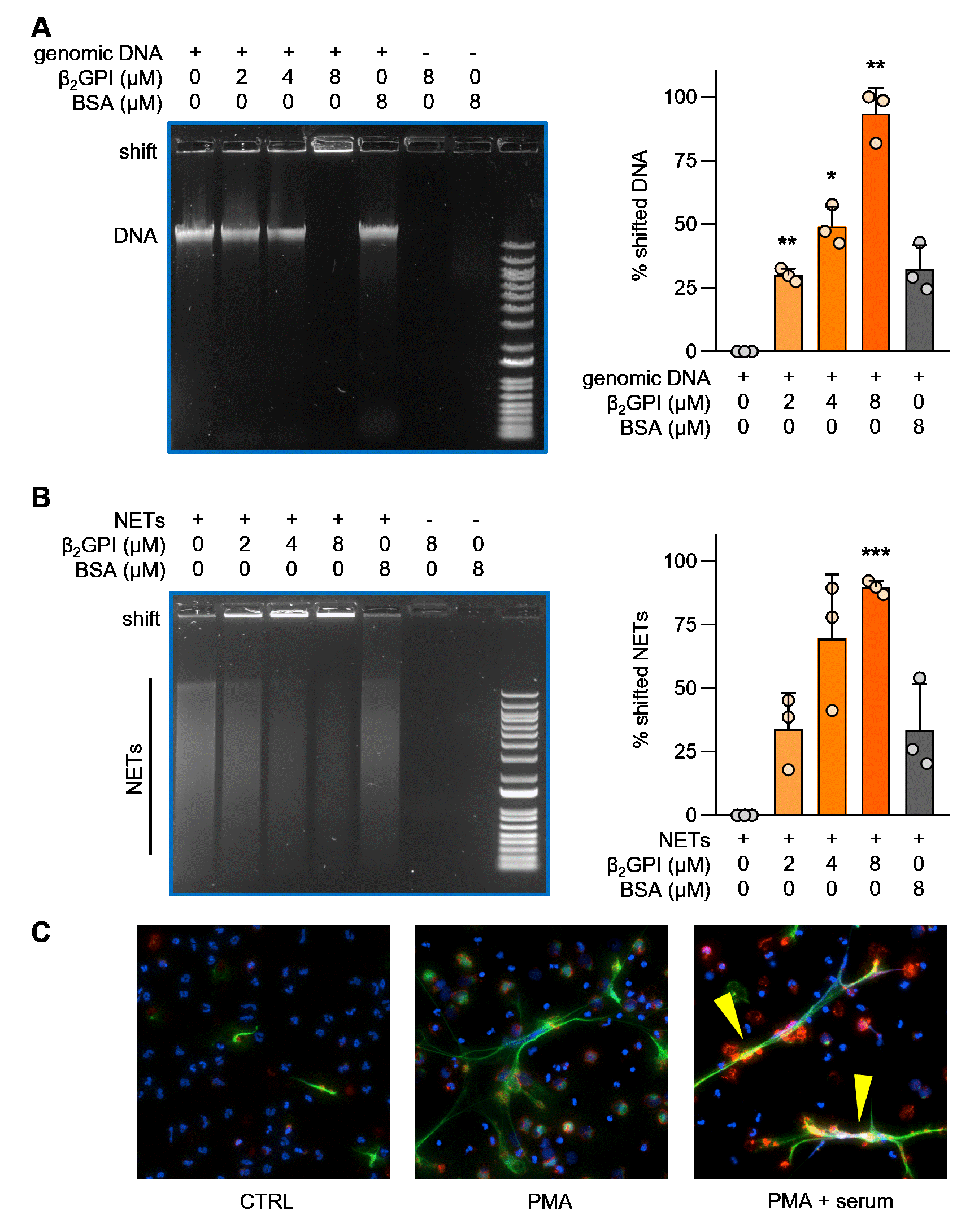 Figure 1: β2GPI binds to DNA and NETs. A-B, Different concentrations of purified β2GPI protein were allowed to form complexes with genomic DNA or NETs as indicated, before resolving the complexes on an agarose gel. Quantification is from three independent experiments; *p < 0.05, **p < 0.01, ***p < 0.001 as compared with the first lane by one-way ANOVA corrected for multiple comparisons. C, Control neutrophils were stimulated with PMA to generate NETs in the presence and absence of 10% human serum (a source of β2GPI) and stained for: DNA=blue, neutrophil elastase=green, β2GPI=red and PMA=phorbol 12-myristate 13-acetate. NETs decorated with β2GPI are indicated by yellow arrows.
Figure 1: β2GPI binds to DNA and NETs. A-B, Different concentrations of purified β2GPI protein were allowed to form complexes with genomic DNA or NETs as indicated, before resolving the complexes on an agarose gel. Quantification is from three independent experiments; *p < 0.05, **p < 0.01, ***p < 0.001 as compared with the first lane by one-way ANOVA corrected for multiple comparisons. C, Control neutrophils were stimulated with PMA to generate NETs in the presence and absence of 10% human serum (a source of β2GPI) and stained for: DNA=blue, neutrophil elastase=green, β2GPI=red and PMA=phorbol 12-myristate 13-acetate. NETs decorated with β2GPI are indicated by yellow arrows.
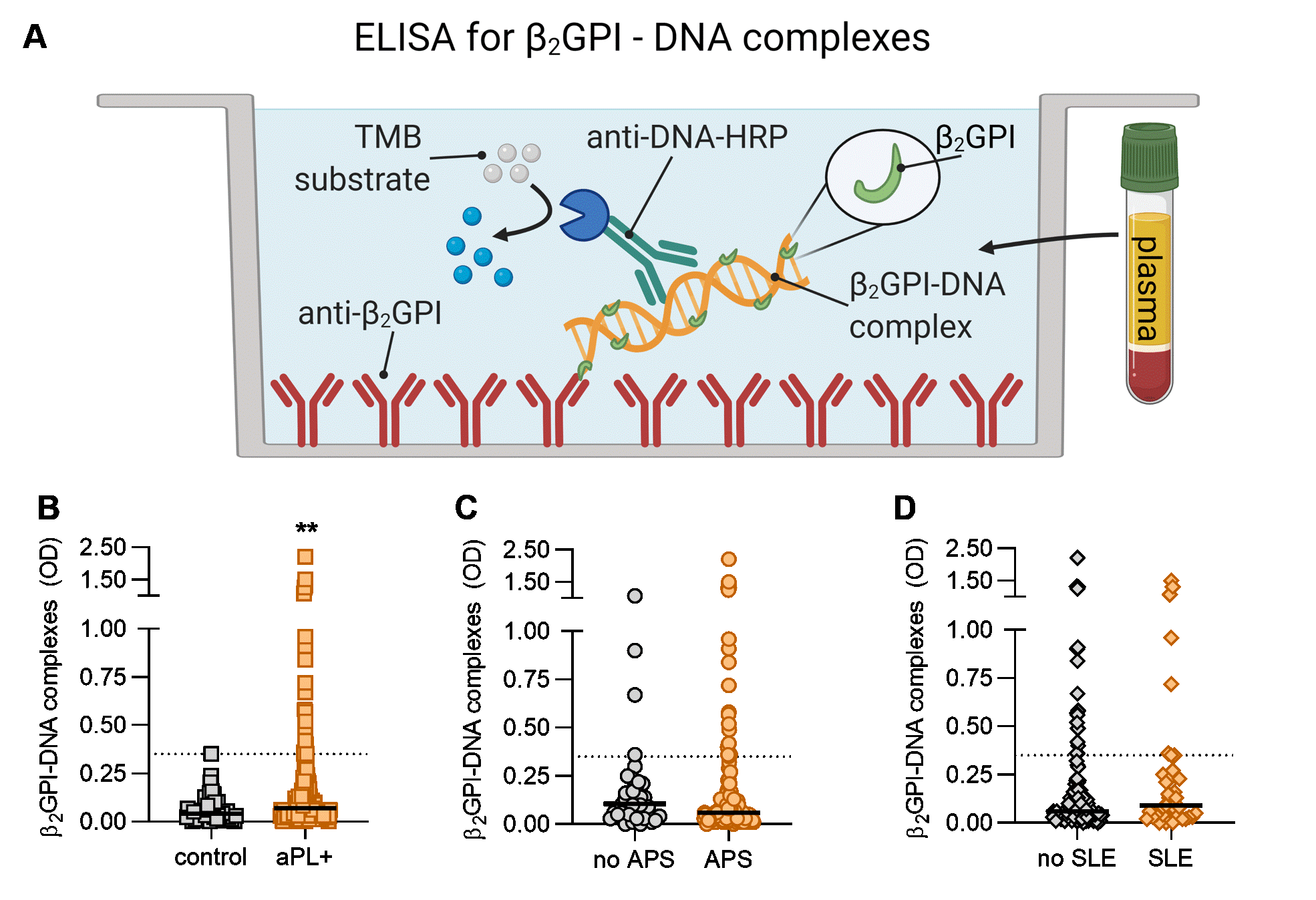 Figure 2: Detection of circulating β2GPI-DNA complexes in antiphospholipid antibody (aPL)-positive patients. A, Schematic illustration of a novel β2GPI -DNA ELISA. B, Levels of β2GPI-DNA were compared between healthy controls (n=52) and aPL-positive patients (n=170, 103 primary APS, 29 secondary APS, and 38 aPL-positive but without criteria APS manifestations). C, β2GPI-DNA in patients meeting classification criteria for APS (n=132) compared with those that do not (n=38). D, Levels of β2GPI-DNA in patients with SLE (n=39) compared with those without SLE (n=131); Mann-Whitney test was used for group comparison, **p < 0.01. OD=optical density. HRP=horse radish peroxidase, TMB=3,3',5,5'-tetramethylbenzidine.
Figure 2: Detection of circulating β2GPI-DNA complexes in antiphospholipid antibody (aPL)-positive patients. A, Schematic illustration of a novel β2GPI -DNA ELISA. B, Levels of β2GPI-DNA were compared between healthy controls (n=52) and aPL-positive patients (n=170, 103 primary APS, 29 secondary APS, and 38 aPL-positive but without criteria APS manifestations). C, β2GPI-DNA in patients meeting classification criteria for APS (n=132) compared with those that do not (n=38). D, Levels of β2GPI-DNA in patients with SLE (n=39) compared with those without SLE (n=131); Mann-Whitney test was used for group comparison, **p < 0.01. OD=optical density. HRP=horse radish peroxidase, TMB=3,3',5,5'-tetramethylbenzidine.
Disclosures: K. Kmetova, None; S. Yalavarthi, None; C. Hoy, None; C. Sarosh, None; N. Peters, None; T. Smith, None; S. Navaz, None; Y. Zuo, None; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb.
Background/Purpose: Beta-2 glycoprotein I (β2GPI) is an abundant plasma protein and a critically important autoantigen in APS. While β2GPI has been reported to have antioxidant, anticoagulant, and scavenging functions, its most important physiological roles continue to be debated. Old but intriguing reports have suggested that β2GPI may function as a DNA-binding protein. Here, we aimed to dissect the interaction between β2GPI and not only DNA, but also neutrophil extracellular traps (NETs). NETs are prothrombotic tangles of nuclear DNA, histones, and granular proteins extruded from activated neutrophils in APS. We also sought to evaluate for the presence and potential clinical significance of β2GPI-DNA complexes in a large cohort of antiphospholipid antibody (aPL)-positive patients.
Methods: We used an electrophoretic mobility shift assay (EMSA) to assess potential direct interaction between β2GPI and either genomic DNA or purified NETs. Such interactions were also assessed by immunofluorescence microscopy after inducing NET formation with phorbol 12-myristate 13-acetate (PMA); this was done in the presence of human serum as a source of β2GPI. Finally, we developed a novel sandwich ELISA to measure circulating β2GPI-DNA complexes in the plasma of patients.
Results: By EMSA, β2GPI caused a dose-dependent retardation of the mobility of both genomic DNA and isolated NETs through an agarose gel (Fig 1A-B). By immunofluorescence microscopy, we observed β2GPI in intimate association with NET strands (Fig 1C). We next evaluated the presence of circulating β2GPI-DNA complexes in the plasma of aPL-positive patients (78% meeting criteria for APS, Fig 2A). As compared with healthy controls, many aPL-positive patients demonstrated elevated levels of β2GPI-DNA complexes (p< 0.01, Fig 2B). No difference was found between patients who met classification criteria for APS and those who did not (p=0.10, Fig 2C). There was also no difference in circulating β2GPI-DNA complexes in the presence or absence of SLE (Fig 2D). Notably, β2GPI-DNA complexes demonstrated a strong positive correlation with neutrophil elastase-DNA complexes (r=0.50, p< 0.001), an established biomarker for NETs. There was a negative correlation with absolute neutrophil count (r=-0.26, p=0.007), suggesting a potential association with neutrophil cell death. We also considered whether β2GPI-DNA correlated with levels of aPL. While no such correlation was appreciated for traditional aPL tests such as anticardiolipin antibodies, anti-β2GPI antibodies, and lupus anticoagulant, we did find a positive correlation with anti-PS/PT IgG (r=0.18, p< 0.05). There was also a non-significant association with anti-dsDNA antibodies (r=0.20, p=0.053).
Conclusion: In summary, our data confirm the direct association of β2GPI with DNA and NETs. Many aPL-positive patients have high levels of circulating β2GPI-DNA complexes, which likely relate to the heightened neutrophil activation and NET formation that are integral to aPL-mediated thromboinflammation. The extent to which the β2GPI-NET interaction may amplify autoantigen spread and perpetuate autoimmunity in APS warrants further investigation.
 Figure 1: β2GPI binds to DNA and NETs. A-B, Different concentrations of purified β2GPI protein were allowed to form complexes with genomic DNA or NETs as indicated, before resolving the complexes on an agarose gel. Quantification is from three independent experiments; *p < 0.05, **p < 0.01, ***p < 0.001 as compared with the first lane by one-way ANOVA corrected for multiple comparisons. C, Control neutrophils were stimulated with PMA to generate NETs in the presence and absence of 10% human serum (a source of β2GPI) and stained for: DNA=blue, neutrophil elastase=green, β2GPI=red and PMA=phorbol 12-myristate 13-acetate. NETs decorated with β2GPI are indicated by yellow arrows.
Figure 1: β2GPI binds to DNA and NETs. A-B, Different concentrations of purified β2GPI protein were allowed to form complexes with genomic DNA or NETs as indicated, before resolving the complexes on an agarose gel. Quantification is from three independent experiments; *p < 0.05, **p < 0.01, ***p < 0.001 as compared with the first lane by one-way ANOVA corrected for multiple comparisons. C, Control neutrophils were stimulated with PMA to generate NETs in the presence and absence of 10% human serum (a source of β2GPI) and stained for: DNA=blue, neutrophil elastase=green, β2GPI=red and PMA=phorbol 12-myristate 13-acetate. NETs decorated with β2GPI are indicated by yellow arrows. Figure 2: Detection of circulating β2GPI-DNA complexes in antiphospholipid antibody (aPL)-positive patients. A, Schematic illustration of a novel β2GPI -DNA ELISA. B, Levels of β2GPI-DNA were compared between healthy controls (n=52) and aPL-positive patients (n=170, 103 primary APS, 29 secondary APS, and 38 aPL-positive but without criteria APS manifestations). C, β2GPI-DNA in patients meeting classification criteria for APS (n=132) compared with those that do not (n=38). D, Levels of β2GPI-DNA in patients with SLE (n=39) compared with those without SLE (n=131); Mann-Whitney test was used for group comparison, **p < 0.01. OD=optical density. HRP=horse radish peroxidase, TMB=3,3',5,5'-tetramethylbenzidine.
Figure 2: Detection of circulating β2GPI-DNA complexes in antiphospholipid antibody (aPL)-positive patients. A, Schematic illustration of a novel β2GPI -DNA ELISA. B, Levels of β2GPI-DNA were compared between healthy controls (n=52) and aPL-positive patients (n=170, 103 primary APS, 29 secondary APS, and 38 aPL-positive but without criteria APS manifestations). C, β2GPI-DNA in patients meeting classification criteria for APS (n=132) compared with those that do not (n=38). D, Levels of β2GPI-DNA in patients with SLE (n=39) compared with those without SLE (n=131); Mann-Whitney test was used for group comparison, **p < 0.01. OD=optical density. HRP=horse radish peroxidase, TMB=3,3',5,5'-tetramethylbenzidine.Disclosures: K. Kmetova, None; S. Yalavarthi, None; C. Hoy, None; C. Sarosh, None; N. Peters, None; T. Smith, None; S. Navaz, None; Y. Zuo, None; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb.

