Back
Poster Session B
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0850–0880) Pediatric Rheumatology – Clinical Poster I: JIA
0863: Effect of Glucocorticoids on Patient Reported Outcomes in Patients Started on a Biologic Consensus Treatment Plan for the "First Line Options for Systemic JIA Treatment" (FROST) Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- KJ
Karen James, MD, MSc
University of Utah
Salt Lake City, UT, United States
Abstract Poster Presenter(s)
Karen James1, George Tomlinson2, Tim Beukelman3, Laura Schanberg4, Anne Dennos5, VIncent Del Gaizo6, Marian Jelinek7, Erin Pfeifer8, Shalini Mohan9 and Yukiko Kimura10, 1University of Utah, Salt Lake City, UT, 2University of Toronto, Toronto, ON, Canada, 3Childhood Arthritis and Rheumatology Research Alliance (CARRA), Birmingham, AL, 4Duke University Medical Center, Durham, NC, 5Duke University, Durham, NC, 6Childhood Arthritis and Rheumatology Research Alliance (CARRA), Durham, NC, 7Childhood Arthritis and Rheumatology Research Alliance (CARRA), Duke, 8Genentech, Englewood, NJ, 9Genentech, San Diego, 10Hackensack Meridian Health, New York, NY
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (sJIA) is a systemic autoinflammatory disease characterized by high fevers, rash and arthritis. Current treatment regimens often involve biologic (anti-IL-1 or anti-IL6) medications, glucocorticoids (GC), or both. Both treatments and disease can have a significant impact on health-related quality of life (HRQoL). GC may be used in addition to biologic treatment for many reasons, including concern that the biologic medication alone may not be enough to control severe systemic disease or pain. This study aimed to evaluate the impact of early use of GC on HRQoL in newly diagnosed sJIA patients started on a biologic treatment.
Methods: Data on patient reported outcomes (PROs) was collected as part of the FiRst-line Options for Systemic juvenile idiopathic arthritis Treatment (FROST) study, a non-randomized prospective observational study of outcomes in children with newly diagnosed sJIA treated by one of 4 Childhood Arthritis and Rheumatology Research Alliance (CARRA) consensus treatment plans (CTPs) for sJIA enrolled in the CARRA Registry. Treatment, including GC use, was at the discretion of the treating provider. This analysis included only subjects treated with a biologic (anti-IL1 or anti-IL6) CTP. Early GC usage was defined as any use reported at the baseline, 2 week, or 1 month visit. Patients were evaluated by their treating physician at baseline, week 2, and months 1, 3, 6, 9, and 12; PROs collected at these visits included patient global disease assessment and Patient Reported Outcomes Measurement Information Systems (PROMIS) short forms. Home PROs on pain, fever, and rash were collected every 2 days for 4 weeks, then weekly for 3 months. Generalized estimating equations and linear mixed effects models were used to compare PROs between GC treatment groups.
Results: 63 of the 73 subjects enrolled in FROST began one of the biologic CTPs. The median age was 7 years (IQR 4.0, 11.3), and 64% were male. 31 (49%) received GC within the first month. Baseline disease characteristics of subjects by GC treatment group are shown in table 1. Subjects treated with GC had a higher median baseline joint count and physician global assessment of disease activity, but a lower CRP and patient global assessment of disease activity. (Table 1). No significant differences were noted in patient reported pain from home over the first 12 weeks in those receiving GC in the first month vs those not (p=0.71, Figure 1), or over the first year of follow-up in terms of PROMIS scores on pain interference (p=0.73), fatigue (p=0.87), mobility(p=0.28), or upper extremity function (p=0.18), after adjusting for time and baseline joint count. (figure 2)
Conclusion: Although GC's were given at the providers' discretion, patients had very similar baseline characteristics regardless of GC treatment group. There were no significant differences in the trend of improvement in most PROs over the first year of treatment, with point estimates for mobility, pain interference, and upper extremity function appearing better in the group not treated with GC, even after adjusting for joint count. This suggests that early GC use, when used with biologics, may not have significant effects in the patient's experience of their disease.
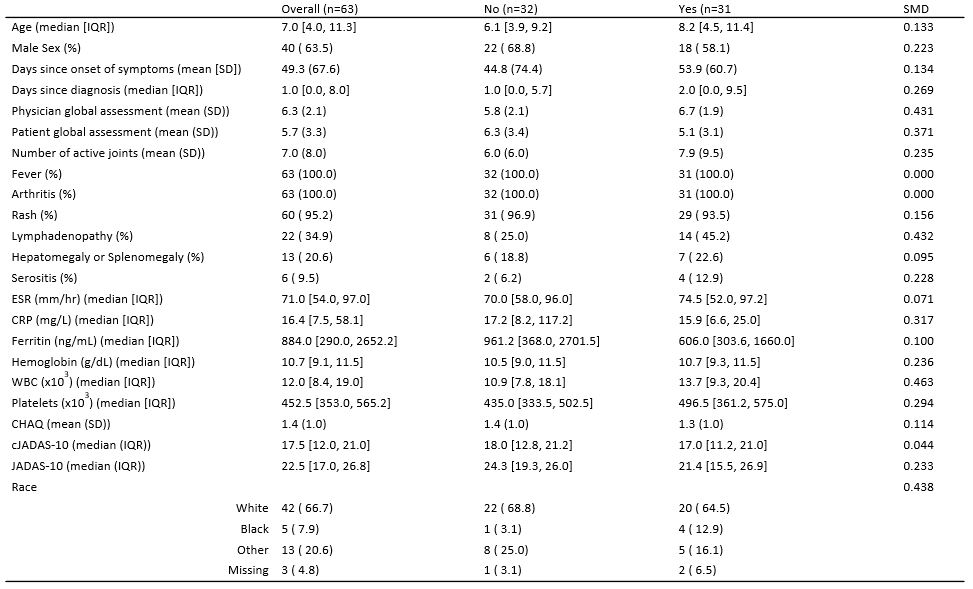 Table 1: Baseline Characteristics of subjects Stratified by any steroid use in the first month vs No steroid use in the first month. cJADAS-10*: Clinical Juvenile Arthritis Disease Activity Score 10; JADAS-10: Juvenile Arthritis Disease Activity Score 10. SMD: standardized mean difference
Table 1: Baseline Characteristics of subjects Stratified by any steroid use in the first month vs No steroid use in the first month. cJADAS-10*: Clinical Juvenile Arthritis Disease Activity Score 10; JADAS-10: Juvenile Arthritis Disease Activity Score 10. SMD: standardized mean difference
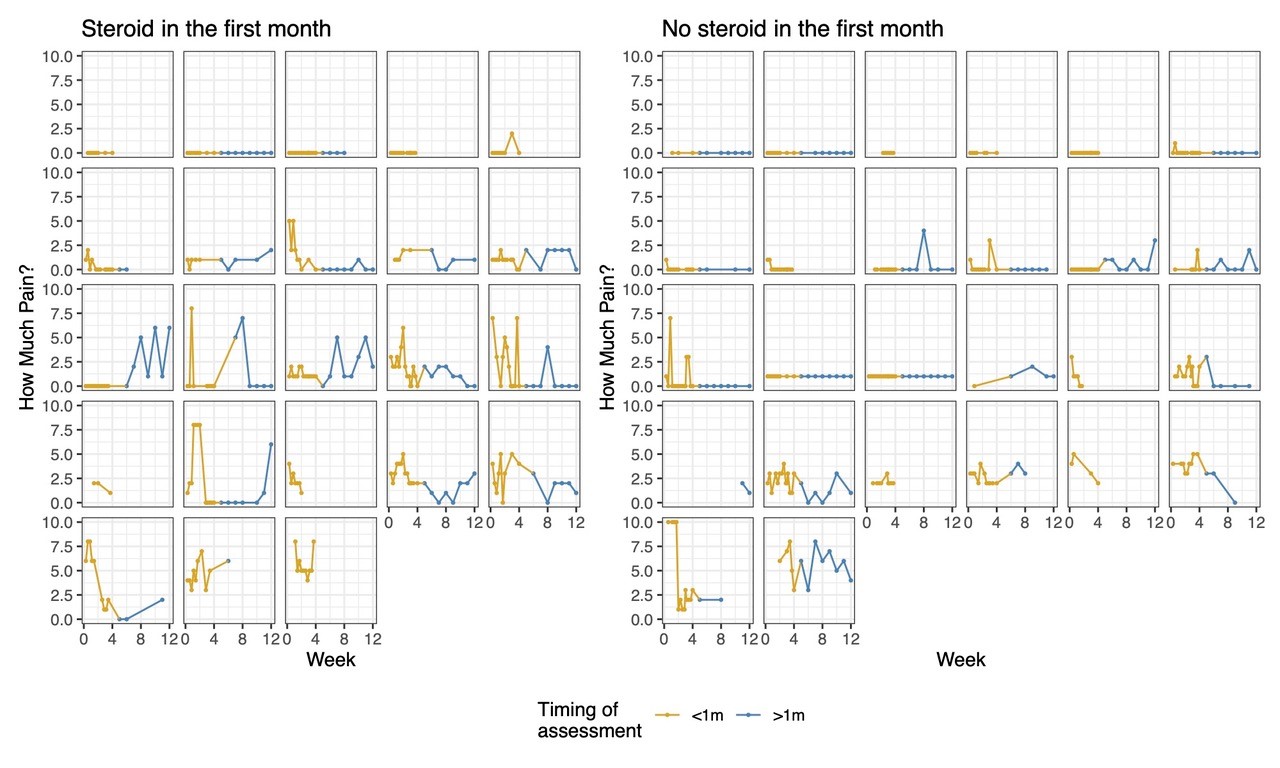 Figure 1. Trends in home reported pain scores of individual patients over the first 12 weeks of enrollment, comparing those who received early glucocorticoids in the first month vs those who did not. The gold represents the first month of data during the exposure period.
Figure 1. Trends in home reported pain scores of individual patients over the first 12 weeks of enrollment, comparing those who received early glucocorticoids in the first month vs those who did not. The gold represents the first month of data during the exposure period.
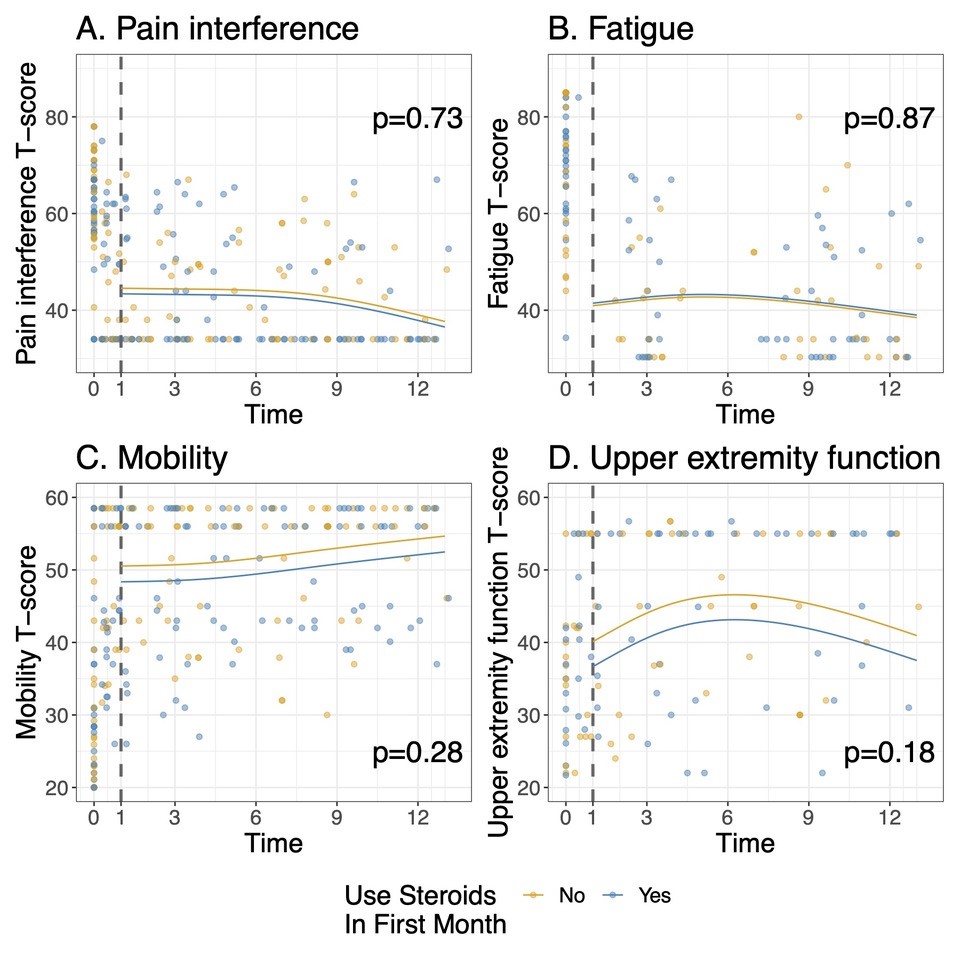 Figure 2: Patient-Reported Outcome Measures Information System Short form scores over the first 12 months. The fitted curves are estimates from a linear mixed model comparing outcomes in months 1-12 according to receipt of GC in the first month. The model has been adjusted for the baseline joint count. A higher T-score in a domain represents more of the domain being measured: for A and B (pain interference and fatigue), lower is better, and for C and D (mobility and upper extremity), higher is better.
Figure 2: Patient-Reported Outcome Measures Information System Short form scores over the first 12 months. The fitted curves are estimates from a linear mixed model comparing outcomes in months 1-12 according to receipt of GC in the first month. The model has been adjusted for the baseline joint count. A higher T-score in a domain represents more of the domain being measured: for A and B (pain interference and fatigue), lower is better, and for C and D (mobility and upper extremity), higher is better.
Disclosures: K. James, None; G. Tomlinson, None; T. Beukelman, Novartis, UCB; L. Schanberg, UCB, Sanofi; A. Dennos, None; V. Del Gaizo, None; M. Jelinek, None; E. Pfeifer, genentech; S. Mohan, genentech; Y. Kimura, Genentech.
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (sJIA) is a systemic autoinflammatory disease characterized by high fevers, rash and arthritis. Current treatment regimens often involve biologic (anti-IL-1 or anti-IL6) medications, glucocorticoids (GC), or both. Both treatments and disease can have a significant impact on health-related quality of life (HRQoL). GC may be used in addition to biologic treatment for many reasons, including concern that the biologic medication alone may not be enough to control severe systemic disease or pain. This study aimed to evaluate the impact of early use of GC on HRQoL in newly diagnosed sJIA patients started on a biologic treatment.
Methods: Data on patient reported outcomes (PROs) was collected as part of the FiRst-line Options for Systemic juvenile idiopathic arthritis Treatment (FROST) study, a non-randomized prospective observational study of outcomes in children with newly diagnosed sJIA treated by one of 4 Childhood Arthritis and Rheumatology Research Alliance (CARRA) consensus treatment plans (CTPs) for sJIA enrolled in the CARRA Registry. Treatment, including GC use, was at the discretion of the treating provider. This analysis included only subjects treated with a biologic (anti-IL1 or anti-IL6) CTP. Early GC usage was defined as any use reported at the baseline, 2 week, or 1 month visit. Patients were evaluated by their treating physician at baseline, week 2, and months 1, 3, 6, 9, and 12; PROs collected at these visits included patient global disease assessment and Patient Reported Outcomes Measurement Information Systems (PROMIS) short forms. Home PROs on pain, fever, and rash were collected every 2 days for 4 weeks, then weekly for 3 months. Generalized estimating equations and linear mixed effects models were used to compare PROs between GC treatment groups.
Results: 63 of the 73 subjects enrolled in FROST began one of the biologic CTPs. The median age was 7 years (IQR 4.0, 11.3), and 64% were male. 31 (49%) received GC within the first month. Baseline disease characteristics of subjects by GC treatment group are shown in table 1. Subjects treated with GC had a higher median baseline joint count and physician global assessment of disease activity, but a lower CRP and patient global assessment of disease activity. (Table 1). No significant differences were noted in patient reported pain from home over the first 12 weeks in those receiving GC in the first month vs those not (p=0.71, Figure 1), or over the first year of follow-up in terms of PROMIS scores on pain interference (p=0.73), fatigue (p=0.87), mobility(p=0.28), or upper extremity function (p=0.18), after adjusting for time and baseline joint count. (figure 2)
Conclusion: Although GC's were given at the providers' discretion, patients had very similar baseline characteristics regardless of GC treatment group. There were no significant differences in the trend of improvement in most PROs over the first year of treatment, with point estimates for mobility, pain interference, and upper extremity function appearing better in the group not treated with GC, even after adjusting for joint count. This suggests that early GC use, when used with biologics, may not have significant effects in the patient's experience of their disease.
 Table 1: Baseline Characteristics of subjects Stratified by any steroid use in the first month vs No steroid use in the first month. cJADAS-10*: Clinical Juvenile Arthritis Disease Activity Score 10; JADAS-10: Juvenile Arthritis Disease Activity Score 10. SMD: standardized mean difference
Table 1: Baseline Characteristics of subjects Stratified by any steroid use in the first month vs No steroid use in the first month. cJADAS-10*: Clinical Juvenile Arthritis Disease Activity Score 10; JADAS-10: Juvenile Arthritis Disease Activity Score 10. SMD: standardized mean difference Figure 1. Trends in home reported pain scores of individual patients over the first 12 weeks of enrollment, comparing those who received early glucocorticoids in the first month vs those who did not. The gold represents the first month of data during the exposure period.
Figure 1. Trends in home reported pain scores of individual patients over the first 12 weeks of enrollment, comparing those who received early glucocorticoids in the first month vs those who did not. The gold represents the first month of data during the exposure period. Figure 2: Patient-Reported Outcome Measures Information System Short form scores over the first 12 months. The fitted curves are estimates from a linear mixed model comparing outcomes in months 1-12 according to receipt of GC in the first month. The model has been adjusted for the baseline joint count. A higher T-score in a domain represents more of the domain being measured: for A and B (pain interference and fatigue), lower is better, and for C and D (mobility and upper extremity), higher is better.
Figure 2: Patient-Reported Outcome Measures Information System Short form scores over the first 12 months. The fitted curves are estimates from a linear mixed model comparing outcomes in months 1-12 according to receipt of GC in the first month. The model has been adjusted for the baseline joint count. A higher T-score in a domain represents more of the domain being measured: for A and B (pain interference and fatigue), lower is better, and for C and D (mobility and upper extremity), higher is better. Disclosures: K. James, None; G. Tomlinson, None; T. Beukelman, Novartis, UCB; L. Schanberg, UCB, Sanofi; A. Dennos, None; V. Del Gaizo, None; M. Jelinek, None; E. Pfeifer, genentech; S. Mohan, genentech; Y. Kimura, Genentech.

