Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1028: How Does PsAID Implementation Affect Treatment Intensification and Patient Satisfaction in PsA?
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CC
Conor Coyle, MBBS, BSc
Oxford University Hospitals NHS Trust
READING, United Kingdom
Abstract Poster Presenter(s)
Conor Coyle1, Caroline Whately-Smith2, Melanie Brooke3, Uta Kiltz4, Ennio Lubrano5, Ruben Queiro6, David Trigos7, Jan Brandt-Juergens8, Salvatore D'Angelo9, Andrea Delle Sedie10, Emmanuelle Dernis11, Philip Helliwell12, Pauline Ho13, Axel Hueber14, BEATRIZ E. JOVEN15, Michaela Koehm16, Carlos Montilla17, Jon Packham18, JOSE PINTO TASENDE19, Felipe Julio Ramirez Garcia20, Adeline Ruyssen-Witrand21, Rossana Scrivo22, sarah twigg23, Martin Welcker24, Theo Wirth25, Laure Gossec26 and Laura Coates27, 1Oxford University Hospitals NHS Trust, Oxford, United Kingdom, 2Whately-Smith Ltd, Kings Langley, United Kingdom, 3Bath Institute for Rheumatic Disease, Bath, United Kingdom, 4Rheumazentrum Ruhrgebiet, Herne, Germany, 5Academic Rheumatology Unit, Dipartimento di Medicina e Scienze della Salute ‘‘Vincenzo Tiberio’’, Università degli Studi del Molise, Campobasso, Italy, 6Faculty of Medicine, Rheumatology Service & the Principality of Asturias Institute for Health Research (ISPA), Universidad de Oviedo, Oveido, Spain, 7Patient partner, Oveido, Spain, 8Rheumatologische Schwerpunktpraxis, Berlin, Germany, 9Rheumatology Department of Lucania - San Carlo Hospital of Potenza, Potenza, Italy, 10University of Pisa, Pisa, Pisa, Italy, 11LE MANS general hospital, LE MANS, France, 12Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom, 13The Kellgren Centre for Rheumatology, Manchester, United Kingdom, 14Paracelsus University Nürnberg, Erlangen, Germany, 15Hospital Universitario 12 de Octubre, Madrid, Spain, 16Rheumatology Goethe-University Frankfurt, Frankfurt Am Main, Germany, 17Complejo asistencial Universitario de Salamanca, Salamanca, Spain, 18University of Nottingham, Stafford, United Kingdom, 19SERGAS, A Coruña, Spain, 20Hospital Clínic, Barcelona, Spain, 21CHU de Toulouse, Toulouse, France, 22Rheumatology Unit, Department of Clinical Internal, Anesthesiological and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy, Roma, Rome, Italy, 23Bradford Teaching hospitals NHS foundation trust, Bradford, United Kingdom, 24GBR, Planegg, Germany, 25Aix Marseille University, Marseille, France, 26Sorbonne Université, Paris, France, 27Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK, Oxford, England, United Kingdom
Background/Purpose: Psoriatic arthritis (PsA) is a heterogenous, multi-dimensional disease. Little is known about factors underpinning treatment intensification in routine practice. Treatment pathways are not well defined and need individual tailoring. The purpose of the ASSIST study was to investigate the prescribing practice for PsA in routine care and whether the use of the patient reported outcome (PRO), PsA Impact of Disease questionnaire (PsAID-12), impacted treatment decisions.
Methods: ASSIST was a cross sectional study of patients diagnosed with PsA (by a rheumatologist using CASPAR criteria). Patients were selected using systematic sampling with random starting numbers generated for each site. Participants were treated in their usual routine clinical practice, decisions on whether treatment was escalated, unchanged or reduced (and why) were recorded. The PsAID-12 questionnaire and additional PROs were provided to patients in one single study visit, during a routine clinic appointment. Univariable and multivariable analyses were performed to explain treatment escalation. There was no imputation of missing data.
Results: 503 patients from 24 centres across five countries in Europe (49.1% F, 50.9% M) aged 18 years or above (mean age 53) participated in the survey between 12/07/2021-22/03/2022. Overall, treatment was changed for 182 patients (36.2%) with an increase in treatment being the most common type of change (160 patients, 31.8%). Treatment escalation was most common in the UK (51 patients, 47.7%) compared to other countries (31.5% of patients or fewer). In 22/24 sites, the mean PsAID score for patients with treatment escalation was higher than that for those with no escalation. (figure 1) The PsAID score was found to have statistically significant association with the odds of treatment escalation (OR: 1.58; p< 0.0001) - the estimated odds of treatment escalation increased by 58% with every 1-point increase in the score. A statistically significant relationship between treatment escalation and patient characteristics, physician characteristics, disease activity, disease impact was identified (Figure 2). Only age, tender joint count and comorbidity index were not significantly associated with treatment escalation. A high level of correlation was seen between a number of these variables, including physician's global assessment of disease and the patient reported PsAID score. In most cases, the clinicians reported that the PsAID score did not significantly influence the decision on treatment escalation.
Conclusion: The PsAID score had a significant association with odds of treatment escalation. The greater the PsAID score, the more likely escalation in treatment. Several factors influenced treatment change indicating the complex decision making in routine clinics. Clinicians reported PsAID score did not impact treatment escalation in most cases, but a significant correlation between PsAID and physician global scores was seen. Our work highlights the influence of multiple factors on decision making when reviewing treatments for individuals with PsA providing insight into the management of patients with this complex condition.
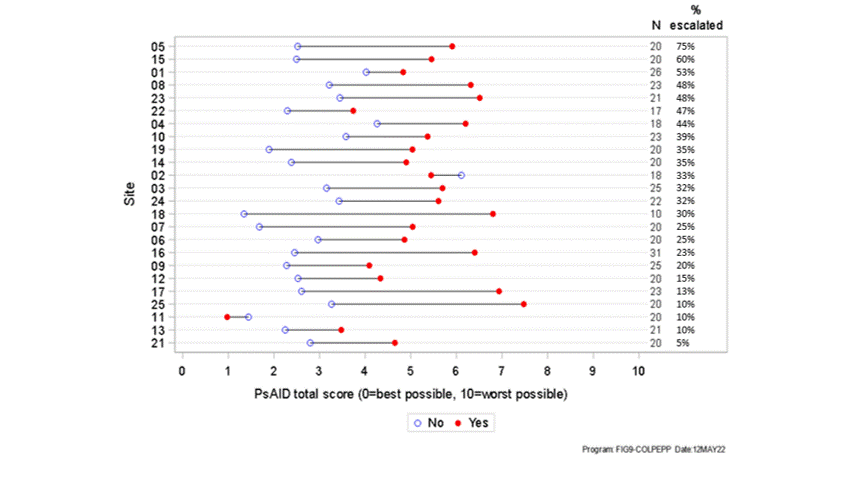 Figure 1: Mean PsAID score by treatment escalation, by site
Figure 1: Mean PsAID score by treatment escalation, by site
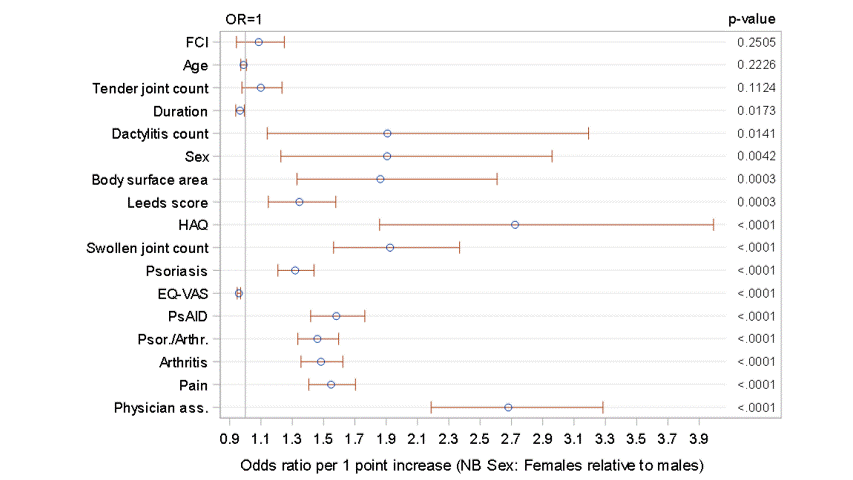 Figure 2: Effect of each variable on the odds of treatment escalation
Figure 2: Effect of each variable on the odds of treatment escalation
Disclosures: C. Coyle, None; C. Whately-Smith, None; M. Brooke, None; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; E. Lubrano, None; R. Queiro, None; D. Trigos, None; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; S. D'Angelo, Merck/MSD, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Novartis, Pfizer, UCB; A. Delle Sedie, None; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis; P. Ho, None; A. Hueber, None; B. JOVEN, Novartis, UCB, Janssen, Amgen, AbbVie/Abbott, Eli Lilly; M. Koehm, Janssen; C. Montilla, None; J. Packham, None; J. PINTO TASENDE, None; F. Ramirez Garcia, Amgen; A. Ruyssen-Witrand, Pfizer, AbbVie/Abbott, Novartis, Eli Lilly, Janssen, Bristol-Myers Squibb(BMS), galapagos, fresenius kabi, Merck/MSD, UCB, Pfizer, Roche, Sanofi; R. Scrivo, None; s. twigg, None; M. Welcker, None; T. Wirth, None; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK).
Background/Purpose: Psoriatic arthritis (PsA) is a heterogenous, multi-dimensional disease. Little is known about factors underpinning treatment intensification in routine practice. Treatment pathways are not well defined and need individual tailoring. The purpose of the ASSIST study was to investigate the prescribing practice for PsA in routine care and whether the use of the patient reported outcome (PRO), PsA Impact of Disease questionnaire (PsAID-12), impacted treatment decisions.
Methods: ASSIST was a cross sectional study of patients diagnosed with PsA (by a rheumatologist using CASPAR criteria). Patients were selected using systematic sampling with random starting numbers generated for each site. Participants were treated in their usual routine clinical practice, decisions on whether treatment was escalated, unchanged or reduced (and why) were recorded. The PsAID-12 questionnaire and additional PROs were provided to patients in one single study visit, during a routine clinic appointment. Univariable and multivariable analyses were performed to explain treatment escalation. There was no imputation of missing data.
Results: 503 patients from 24 centres across five countries in Europe (49.1% F, 50.9% M) aged 18 years or above (mean age 53) participated in the survey between 12/07/2021-22/03/2022. Overall, treatment was changed for 182 patients (36.2%) with an increase in treatment being the most common type of change (160 patients, 31.8%). Treatment escalation was most common in the UK (51 patients, 47.7%) compared to other countries (31.5% of patients or fewer). In 22/24 sites, the mean PsAID score for patients with treatment escalation was higher than that for those with no escalation. (figure 1) The PsAID score was found to have statistically significant association with the odds of treatment escalation (OR: 1.58; p< 0.0001) - the estimated odds of treatment escalation increased by 58% with every 1-point increase in the score. A statistically significant relationship between treatment escalation and patient characteristics, physician characteristics, disease activity, disease impact was identified (Figure 2). Only age, tender joint count and comorbidity index were not significantly associated with treatment escalation. A high level of correlation was seen between a number of these variables, including physician's global assessment of disease and the patient reported PsAID score. In most cases, the clinicians reported that the PsAID score did not significantly influence the decision on treatment escalation.
Conclusion: The PsAID score had a significant association with odds of treatment escalation. The greater the PsAID score, the more likely escalation in treatment. Several factors influenced treatment change indicating the complex decision making in routine clinics. Clinicians reported PsAID score did not impact treatment escalation in most cases, but a significant correlation between PsAID and physician global scores was seen. Our work highlights the influence of multiple factors on decision making when reviewing treatments for individuals with PsA providing insight into the management of patients with this complex condition.
 Figure 1: Mean PsAID score by treatment escalation, by site
Figure 1: Mean PsAID score by treatment escalation, by site Figure 2: Effect of each variable on the odds of treatment escalation
Figure 2: Effect of each variable on the odds of treatment escalationDisclosures: C. Coyle, None; C. Whately-Smith, None; M. Brooke, None; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; E. Lubrano, None; R. Queiro, None; D. Trigos, None; J. Brandt-Juergens, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Pfizer, Roche, UCB, Sanofi-Aventis, Medac, Gilead, Gilead, Affibody; S. D'Angelo, Merck/MSD, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Novartis, Pfizer, UCB; A. Delle Sedie, None; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis; P. Ho, None; A. Hueber, None; B. JOVEN, Novartis, UCB, Janssen, Amgen, AbbVie/Abbott, Eli Lilly; M. Koehm, Janssen; C. Montilla, None; J. Packham, None; J. PINTO TASENDE, None; F. Ramirez Garcia, Amgen; A. Ruyssen-Witrand, Pfizer, AbbVie/Abbott, Novartis, Eli Lilly, Janssen, Bristol-Myers Squibb(BMS), galapagos, fresenius kabi, Merck/MSD, UCB, Pfizer, Roche, Sanofi; R. Scrivo, None; s. twigg, None; M. Welcker, None; T. Wirth, None; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK).

