Back
Poster Session B
Vasculitis
Session: (1071–1093) Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
1087: Identification of Novel Proteomic Biomarkers of Disease Activity in ANCA-Associated Vasculitis Using a High Throughput Approach
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- ZW
Zachary Wallace, MD, MSc
Massachusetts General Hospital
Newton, MA, United States
Abstract Poster Presenter(s)
Zachary Wallace1, Amit Joshi1, Xiaoqing Fu1, Claire Cook1, yuqing zhang2 and Hyon Choi3, 1Massachusetts General Hospital, Boston, MA, 2Massachusetts General Hospital, Quincy, MA, 3MASSACHUSETTS GENERAL HOSPITAL, Lexington, MA
Background/Purpose: ANCA-associated vasculitis (AAV) is associated with excess morbidity and mortality. Identifying novel biomarkers may reveal new therapeutic targets and clinically useful biomarkers of disease activity to personalize management. ANCA is the most used biomarker of disease activity in AAV but poorly predicts future disease activity. Previous studies investigated protein signatures of disease activity using a small panel of candidate markers. We leveraged a high-throughput approach to identify potential biomarkers in AAV.
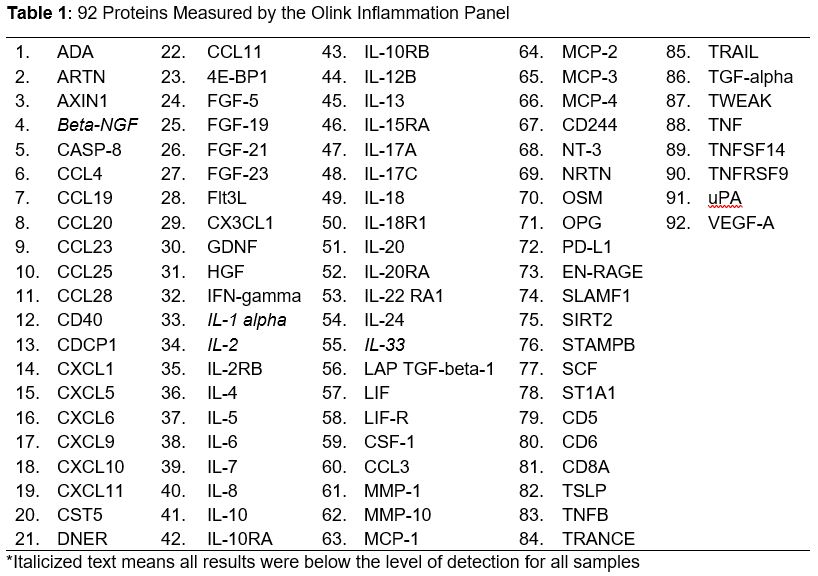
Methods: Serum samples from patients in the Mass General Brigham (MGB) AAV cohort were retrieved from the MGB Biobank or a prospective biorepository. We chose a random 78 who had ANCA+ AAV. We classified disease activity (Active/Remission) at sample collection. We determined whether a clinical ELISA test for MPO- or PR3-ANCA was performed when the sample was collected; if so, we extracted the titer and reference ranges. We used the Olink high-throughput assay for 92 inflammatory proteins (Table 1). We compared the concentration of each protein among (1) active disease vs remission; (2) ANCA positive vs negative; and (3) ANCA positive vs negative among those in remission. We used unadjusted and age- and sex-adjusted linear regression to compare normalized protein levels across groups. Adjustment for multiple comparisons was based on the 10% false discovery rate (FDR) procedure.
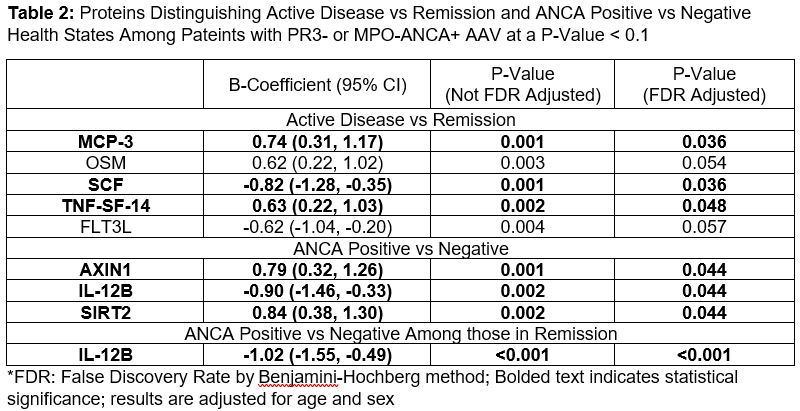
Results: Of 78 subjects, the mean age was 56.8 (SD17.6), 56% were female, 44% were MPO-ANCA+, and 28% had active disease at sample collection. 65 patients had an ANCA ELISA available, 45 of whom were in remission. Of the 92 proteins assessed, 88 were analyzed. After adjusting for multiple comparisons (Table 2), three proteins differentiated those with active disease from those in remission: monocyte chemotactant protein (MCP)-3 (adjusted B coefficient 0.74 (95%CI 0.31 to 1.17), FDR-adjusted p=0.036), stem cell factor (SCF, -0.82 (95%CI -1.28 to -0.35), p=0.036), and tumor necrosis factor superfamily member (TNF-SF)-14 (0.63 (0.22 to 1.03), p=0.048). Three proteins differentiated patients with a positive ANCA vs negative ANCA: Axin 1 (0.79 (0.32 to 1.26), p=0.04), Interleukin (IL)-12B (-0.90 (-1.46 to -0.33), p=0.04), and Sirtuin (SIRT)-2 (0.79 (0.30 to 1.29), p=0.04). Among those in remission, those with a positive ANCA were distinguished from those with a negative ANCA by IL-12B (-1.02 (95%CI -1.55 to -0.49), p=< 0.001).
Conclusion: Using a high-throughput approach, we identified novel candidate markers differentiating active disease from remission and positive vs negative ANCA. These proteins reflect diverse aspects of the immune response. The potential for biomarkers that distinguish ANCA positive from negative status, especially among people in remission, suggests that patients in clinical remission with persistently positive ANCA may have ongoing inflammation that could contribute to subclinical damage. Indeed, a prior study found that IL-12 production by dendritic cells was decreased in patients with active vs remission AAV. These findings require validation in additional cohorts, using longitudinal serial samples, and confirmatory methods.
Disclosures: Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon; A. Joshi, Regeneron Inc; X. Fu, None; C. Cook, None; y. zhang, None; H. Choi, Horizon, Allena, LG, Protalix.
Background/Purpose: ANCA-associated vasculitis (AAV) is associated with excess morbidity and mortality. Identifying novel biomarkers may reveal new therapeutic targets and clinically useful biomarkers of disease activity to personalize management. ANCA is the most used biomarker of disease activity in AAV but poorly predicts future disease activity. Previous studies investigated protein signatures of disease activity using a small panel of candidate markers. We leveraged a high-throughput approach to identify potential biomarkers in AAV.
Methods: Serum samples from patients in the Mass General Brigham (MGB) AAV cohort were retrieved from the MGB Biobank or a prospective biorepository. We chose a random 78 who had ANCA+ AAV. We classified disease activity (Active/Remission) at sample collection. We determined whether a clinical ELISA test for MPO- or PR3-ANCA was performed when the sample was collected; if so, we extracted the titer and reference ranges. We used the Olink high-throughput assay for 92 inflammatory proteins (Table 1). We compared the concentration of each protein among (1) active disease vs remission; (2) ANCA positive vs negative; and (3) ANCA positive vs negative among those in remission. We used unadjusted and age- and sex-adjusted linear regression to compare normalized protein levels across groups. Adjustment for multiple comparisons was based on the 10% false discovery rate (FDR) procedure.
Results: Of 78 subjects, the mean age was 56.8 (SD17.6), 56% were female, 44% were MPO-ANCA+, and 28% had active disease at sample collection. 65 patients had an ANCA ELISA available, 45 of whom were in remission. Of the 92 proteins assessed, 88 were analyzed. After adjusting for multiple comparisons (Table 2), three proteins differentiated those with active disease from those in remission: monocyte chemotactant protein (MCP)-3 (adjusted B coefficient 0.74 (95%CI 0.31 to 1.17), FDR-adjusted p=0.036), stem cell factor (SCF, -0.82 (95%CI -1.28 to -0.35), p=0.036), and tumor necrosis factor superfamily member (TNF-SF)-14 (0.63 (0.22 to 1.03), p=0.048). Three proteins differentiated patients with a positive ANCA vs negative ANCA: Axin 1 (0.79 (0.32 to 1.26), p=0.04), Interleukin (IL)-12B (-0.90 (-1.46 to -0.33), p=0.04), and Sirtuin (SIRT)-2 (0.79 (0.30 to 1.29), p=0.04). Among those in remission, those with a positive ANCA were distinguished from those with a negative ANCA by IL-12B (-1.02 (95%CI -1.55 to -0.49), p=< 0.001).
Conclusion: Using a high-throughput approach, we identified novel candidate markers differentiating active disease from remission and positive vs negative ANCA. These proteins reflect diverse aspects of the immune response. The potential for biomarkers that distinguish ANCA positive from negative status, especially among people in remission, suggests that patients in clinical remission with persistently positive ANCA may have ongoing inflammation that could contribute to subclinical damage. Indeed, a prior study found that IL-12 production by dendritic cells was decreased in patients with active vs remission AAV. These findings require validation in additional cohorts, using longitudinal serial samples, and confirmatory methods.


Disclosures: Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon; A. Joshi, Regeneron Inc; X. Fu, None; C. Cook, None; y. zhang, None; H. Choi, Horizon, Allena, LG, Protalix.

