Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0797: Increased Duration of Mycophenolate Hold Improves Antibody Response to SARS-CoV-2 Vaccination in Patients with Rheumatic and Musculoskeletal Disease
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- SF
Sarah Frey, BS
Johns Hopkins University School of Medicine
Baltimore, MD, United States
Abstract Poster Presenter(s)
Sarah Frey1, Caoilfhionn Connolly1, Teresa Po-Yu Chiang1, Mayan Teles1, Jennifer Alejo1, Jemima Albayda1, Lisa Christopher-Stine1, Dorry Segev2, William Werbel1 and Julie Paik1, 1Johns Hopkins University, Baltimore, MD, 2NYU Langone Health, New York, NY
Background/Purpose: Patients with rheumatic and musculoskeletal diseases (RMD) treated with mycophenolate (MMF) have an attenuated humoral response to SARS-CoV-2 mRNA vaccination. A temporary hold of mycophenolate in the perivaccination period is associated with improved antibody response, prompting the American College of Rheumatology to recommend holding mycophenolate for one week after vaccine dose to enhance immunogenicity. The optimal duration of withholding therapy remains unknown. We sought to understand the impact of the duration of perivaccination mycophenolate hold on disease control (i.e. flare) and immunogenicity of SARS-CoV-2 vaccination in patients with RMD.
Methods: We leveraged an observational prospective cohort of RMD patients without prior COVID-19 who had received two mRNA SARS-CoV-2 vaccine doses. Demographic and clinical information was collected via electronic questionnaire. We included participants who reported MMF use. Serologic testing was undertaken on the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay (range 0.4-250U/mL, later expanded to >2500U/mL) at 2 weeks, 1 month and 3 months after dose 2 (D2); we utilized the highest available titer from any of these time points. Antibody response >250U/mL was defined as high titer. One-month post-D2, participants were invited to complete an online questionnaire detailing prior history of flare, incident flare events including symptoms, duration, and treatment; flare events were qualified as flares requiring treatment by a physician. Characteristics were compared using Wilcoxon rank-sum or Fisher's exact test as appropriate; all tests were two-sided with an α level of 0.05. Analysis was conducted on Stata/SE17.0 (College Station, TX).
Results: Of 220 participants who reported MMF use, 177 (80.5%) did not withhold in the perivaccination period (Table 1). Among the 43 who did withhold MMF, 21 (48.8%) held for less than 10 days, while 22 (51.2%) held for at least 10 days. The median (IQR) days held in each group was 5 (2,7) and 21 (14, 30), respectively. A higher proportion of participants who held for at least ten days had a high antibody response compared to those who continued therapy or held for less than 10 days (64% [14/22] versus 29%[6/21] and 29% [51/177] respectively; p=0.006) (Table 2). There were similar rates of flare in the post-vaccination period in all groups (6.8%, 9.5%, and 4.5% respectively; p=1.00); none reported flare requiring intravenous therapy or hospital admission.
Conclusion: Longer duration of peri-vaccination mycophenolate hold is significantly associated with increased rates of high positive antibody response following two-dose SARS-CoV-2 vaccination. Reassuringly, there was not an increased rate of flare among participants who withheld therapy.
.jpg) Table 1. Demographic and clinical characteristics, stratified by peri-vaccination mycophenolate hold duration.
Table 1. Demographic and clinical characteristics, stratified by peri-vaccination mycophenolate hold duration.
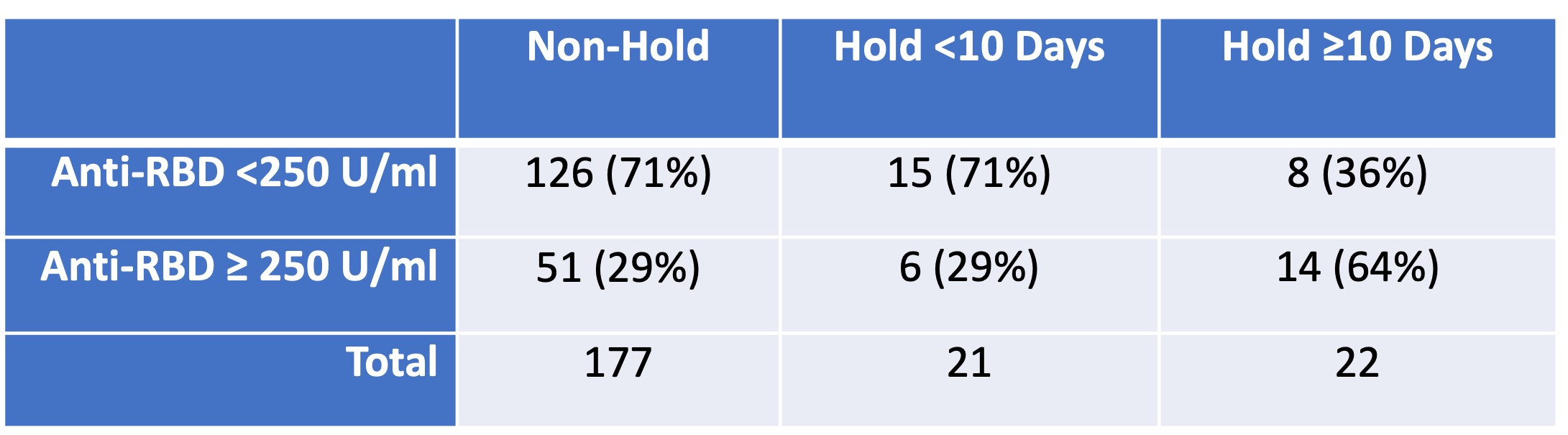 Table 2. Anti-spike antibody response following two dose SARS-CoV-2 vaccine dose in RMD, stratified by peri-vaccination mycophenolate hold duration.
Table 2. Anti-spike antibody response following two dose SARS-CoV-2 vaccine dose in RMD, stratified by peri-vaccination mycophenolate hold duration.
Disclosures: S. Frey, None; C. Connolly, None; T. Po-Yu Chiang, None; M. Teles, None; J. Alejo, None; J. Albayda, None; L. Christopher-Stine, Janssen, Boehringer-Ingelheim, Mallinckroft, EMD-Serono, Allogene, ArgenX; D. Segev, Sanofi, Novartis, Veloxis, Mallinckrodt, Jazz Pharmaceuticals, CSL Behring, Thermo Fisher Scientific, Caredx, Transmedics, Kamada, MediGO, Regeneron, AstraZeneca, Takeda, Bridge to Life; W. Werbel, None; J. Paik, Pfizer Inc, Kezar Inc, Roivant, Argenx, Alexion Inc.
Background/Purpose: Patients with rheumatic and musculoskeletal diseases (RMD) treated with mycophenolate (MMF) have an attenuated humoral response to SARS-CoV-2 mRNA vaccination. A temporary hold of mycophenolate in the perivaccination period is associated with improved antibody response, prompting the American College of Rheumatology to recommend holding mycophenolate for one week after vaccine dose to enhance immunogenicity. The optimal duration of withholding therapy remains unknown. We sought to understand the impact of the duration of perivaccination mycophenolate hold on disease control (i.e. flare) and immunogenicity of SARS-CoV-2 vaccination in patients with RMD.
Methods: We leveraged an observational prospective cohort of RMD patients without prior COVID-19 who had received two mRNA SARS-CoV-2 vaccine doses. Demographic and clinical information was collected via electronic questionnaire. We included participants who reported MMF use. Serologic testing was undertaken on the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay (range 0.4-250U/mL, later expanded to >2500U/mL) at 2 weeks, 1 month and 3 months after dose 2 (D2); we utilized the highest available titer from any of these time points. Antibody response >250U/mL was defined as high titer. One-month post-D2, participants were invited to complete an online questionnaire detailing prior history of flare, incident flare events including symptoms, duration, and treatment; flare events were qualified as flares requiring treatment by a physician. Characteristics were compared using Wilcoxon rank-sum or Fisher's exact test as appropriate; all tests were two-sided with an α level of 0.05. Analysis was conducted on Stata/SE17.0 (College Station, TX).
Results: Of 220 participants who reported MMF use, 177 (80.5%) did not withhold in the perivaccination period (Table 1). Among the 43 who did withhold MMF, 21 (48.8%) held for less than 10 days, while 22 (51.2%) held for at least 10 days. The median (IQR) days held in each group was 5 (2,7) and 21 (14, 30), respectively. A higher proportion of participants who held for at least ten days had a high antibody response compared to those who continued therapy or held for less than 10 days (64% [14/22] versus 29%[6/21] and 29% [51/177] respectively; p=0.006) (Table 2). There were similar rates of flare in the post-vaccination period in all groups (6.8%, 9.5%, and 4.5% respectively; p=1.00); none reported flare requiring intravenous therapy or hospital admission.
Conclusion: Longer duration of peri-vaccination mycophenolate hold is significantly associated with increased rates of high positive antibody response following two-dose SARS-CoV-2 vaccination. Reassuringly, there was not an increased rate of flare among participants who withheld therapy.
.jpg) Table 1. Demographic and clinical characteristics, stratified by peri-vaccination mycophenolate hold duration.
Table 1. Demographic and clinical characteristics, stratified by peri-vaccination mycophenolate hold duration. Table 2. Anti-spike antibody response following two dose SARS-CoV-2 vaccine dose in RMD, stratified by peri-vaccination mycophenolate hold duration.
Table 2. Anti-spike antibody response following two dose SARS-CoV-2 vaccine dose in RMD, stratified by peri-vaccination mycophenolate hold duration. Disclosures: S. Frey, None; C. Connolly, None; T. Po-Yu Chiang, None; M. Teles, None; J. Alejo, None; J. Albayda, None; L. Christopher-Stine, Janssen, Boehringer-Ingelheim, Mallinckroft, EMD-Serono, Allogene, ArgenX; D. Segev, Sanofi, Novartis, Veloxis, Mallinckrodt, Jazz Pharmaceuticals, CSL Behring, Thermo Fisher Scientific, Caredx, Transmedics, Kamada, MediGO, Regeneron, AstraZeneca, Takeda, Bridge to Life; W. Werbel, None; J. Paik, Pfizer Inc, Kezar Inc, Roivant, Argenx, Alexion Inc.

