Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0724–0751) Health Services Research Poster II
0733: Median Patient Global Assessment Varies Far More According to FAST4 (fibromyalgia Assessment Screening Tool) and MDS2 (MDHAQ Depression Screen) on a Single MDHAQ (multidimensional Health Assessment Questionnaire) Than According to Primary Diagnosis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- TP
Theodore Pincus, MD
Rush University Medical Center
Chicago, IL, United States
Abstract Poster Presenter(s)
Theodore Pincus1, Kathryn Gibson2, Juan Schmukler1, Tengfei Li3 and Joel Block1, 1Rush University Medical Center, Chicago, IL, 2Liverpool Hospital, Sydney, Australia, 3Georgetown University, Washington, DC
Background/Purpose: Patient global assessment (PATGL) is a component of DAS28 (disease activity score 28) and CDAI (clinical disease activity index) to assess patients with rheumatoid arthritis (RA), ASDAS (ankylosing spondylitis disease activity score) in axial spondyloarthritis (AxSpA), DAPSA (disease activity in psoriatic arthritis) in psoriatic arthritis (PsA), and other indices. PATGL generally reflects inflammatory activity in selected patients in clinical trials, who may, however, be fewer than 20% of all patients in routine care. PATGL and index scores may be elevated in the absence of inflammation due to fibromyalgia (FM) and/or depression (DEP). These conditions may be easily diagnosed but are often underrecognized. A feasible quantitative tool to identify FM or DEP could clarify interpretation of high PATGL and index scores in routine care, e.g., for treat-to-target, is available from 2 validated indices within a single multidimensional health assessment questionnaire (MDHAQ), fibromyalgia assessment screening tool (FAST4) which agrees ~90% with 2011 revised FM criteria, and MDS2 (MDHAQ depression screen) agrees ~80% with reference HADS-D (hospital anxiety and depression scale–DEP). We analyzed patients seen in routine rheumatology care at two settings on different continents according to FAST4 and MDS2
Methods: The MDHAQ is a 2-page patient self-report questionnaire administered to all patients with all diagnoses in routine rheumatology care at two sites. The MDHAQ includes a 0-10 pain VNS, 0-10 fatigue VNS, 0-3.3 DEP query, self-report 0-54 patient painful joint count, 60-symptom checklist including DEP, medical history queries, and FAST4 and MDS2 indices. FAST4 indicates FM if 3/4 of: pain VNS ≥6/10, fatigue VNS ≥6/10, self-report painful joint count ≥16/54, and/or symptom checklist ≥16/60. MDS2 indicates DEP if 0-3.3 DEP response is ≥2.2 OR positive DEP on the symptom checklist. Median and interquartile range (IQR) for PATGL was computed according to primary diagnoses in patients who were FAST4-/MDS2-, FAST4+/MDS2-, FAST4-/MDS2+, and FAST4+/MDS2.
Results: Median PATGL in 928 unselected patients was 5.0 at both sites (range of medians of specific diagnoses 3.0-6.5, other than primary FM) (Table), higher in non-inflammatory vs inflammatory diagnoses, e.g, 5.0 at both sites in RA and 6.0-6.5 in OA (Table). Median PATGL was 3.0 and 3.3 in FAST4-/MDS2- patients (range 2.0-4.5 except primary FM), 5.0 in FAST4/MDS2+ (range 3.0-6.0 except if only 1 patient in category), 8.0 in FAST4+/MDS2- (range 6.5-9.0), and 8.0 in FAST4+/MDS2+ patients (range 6.5-10.0), similar at both sites.
Conclusion: Median 0-10 PATGL was 2-3-fold higher in FAST4+/MDS2+ patients than in FAST4-/MDS2-. Similar patterns were seen with most diagnoses other than for FM in primary FM, higher in non-inflammatory than in inflammatory diagnoses. These patterns were similar patterns at 2 independent sites on 2 continents. Differences according to FAST4 and MDS2 to screen for FM and DEP were considerably greater than according to any primary diagnosis, feasibly recognized on a single MDHAQ.
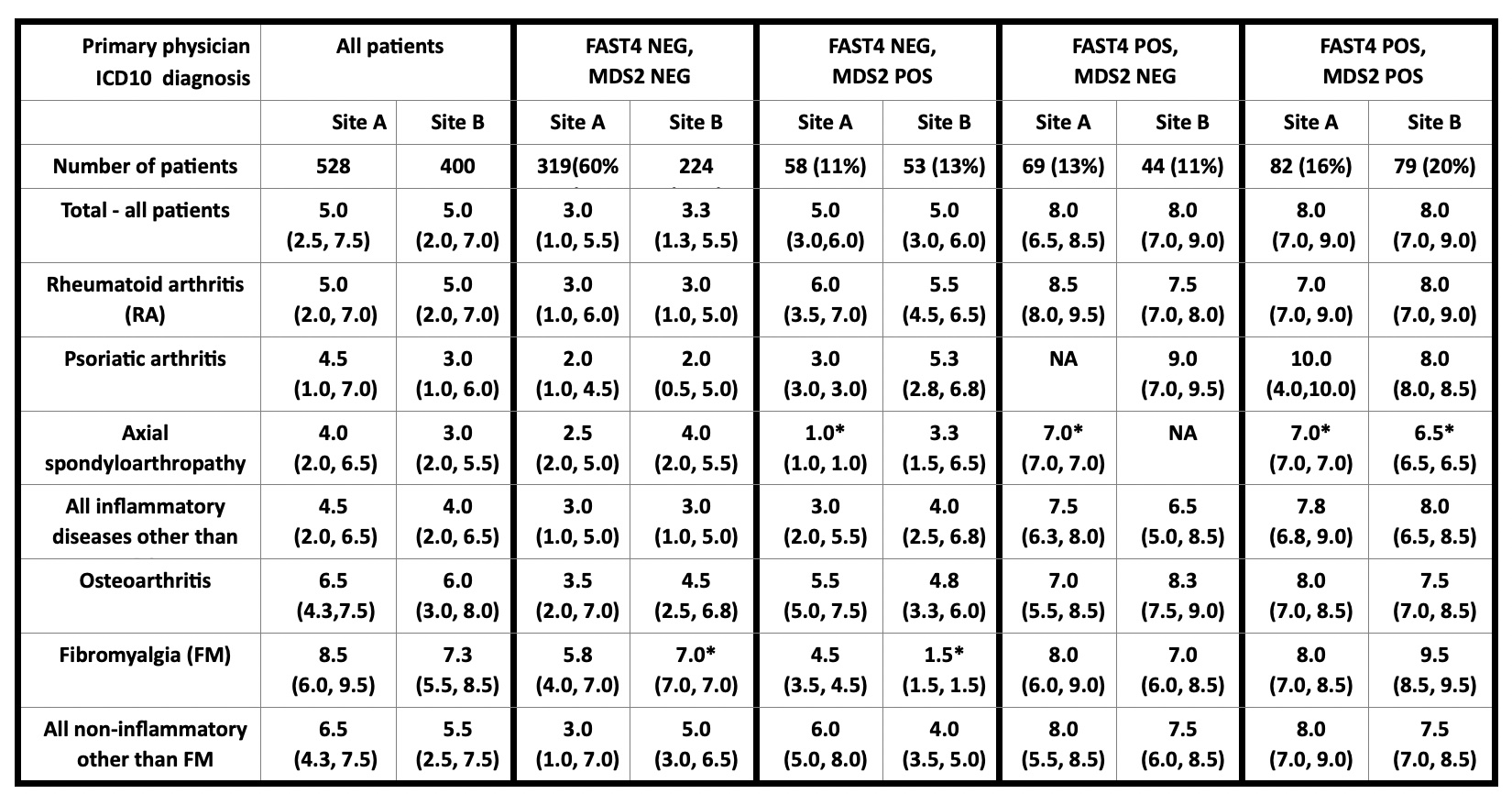 Table: Median patient global assessment in 928 patients with various rheumatic diagnoses at 2 sites, according to 2 feasibly collected and scored indices within a multidimensional health assessment questionnaire (MDHAQ), fibromyalgia assessment screening tool (FAST4) and MDHAQ depression screen (MDS2)
Table: Median patient global assessment in 928 patients with various rheumatic diagnoses at 2 sites, according to 2 feasibly collected and scored indices within a multidimensional health assessment questionnaire (MDHAQ), fibromyalgia assessment screening tool (FAST4) and MDHAQ depression screen (MDS2)
*=Only 1 patient in category, NA=No patient in category
Disclosures: T. Pincus, Medical History Services, LLC, Medical History Services LLC; K. Gibson, Eli Lilly Pty Ltd; J. Schmukler, None; T. Li, None; J. Block, None.
Background/Purpose: Patient global assessment (PATGL) is a component of DAS28 (disease activity score 28) and CDAI (clinical disease activity index) to assess patients with rheumatoid arthritis (RA), ASDAS (ankylosing spondylitis disease activity score) in axial spondyloarthritis (AxSpA), DAPSA (disease activity in psoriatic arthritis) in psoriatic arthritis (PsA), and other indices. PATGL generally reflects inflammatory activity in selected patients in clinical trials, who may, however, be fewer than 20% of all patients in routine care. PATGL and index scores may be elevated in the absence of inflammation due to fibromyalgia (FM) and/or depression (DEP). These conditions may be easily diagnosed but are often underrecognized. A feasible quantitative tool to identify FM or DEP could clarify interpretation of high PATGL and index scores in routine care, e.g., for treat-to-target, is available from 2 validated indices within a single multidimensional health assessment questionnaire (MDHAQ), fibromyalgia assessment screening tool (FAST4) which agrees ~90% with 2011 revised FM criteria, and MDS2 (MDHAQ depression screen) agrees ~80% with reference HADS-D (hospital anxiety and depression scale–DEP). We analyzed patients seen in routine rheumatology care at two settings on different continents according to FAST4 and MDS2
Methods: The MDHAQ is a 2-page patient self-report questionnaire administered to all patients with all diagnoses in routine rheumatology care at two sites. The MDHAQ includes a 0-10 pain VNS, 0-10 fatigue VNS, 0-3.3 DEP query, self-report 0-54 patient painful joint count, 60-symptom checklist including DEP, medical history queries, and FAST4 and MDS2 indices. FAST4 indicates FM if 3/4 of: pain VNS ≥6/10, fatigue VNS ≥6/10, self-report painful joint count ≥16/54, and/or symptom checklist ≥16/60. MDS2 indicates DEP if 0-3.3 DEP response is ≥2.2 OR positive DEP on the symptom checklist. Median and interquartile range (IQR) for PATGL was computed according to primary diagnoses in patients who were FAST4-/MDS2-, FAST4+/MDS2-, FAST4-/MDS2+, and FAST4+/MDS2.
Results: Median PATGL in 928 unselected patients was 5.0 at both sites (range of medians of specific diagnoses 3.0-6.5, other than primary FM) (Table), higher in non-inflammatory vs inflammatory diagnoses, e.g, 5.0 at both sites in RA and 6.0-6.5 in OA (Table). Median PATGL was 3.0 and 3.3 in FAST4-/MDS2- patients (range 2.0-4.5 except primary FM), 5.0 in FAST4/MDS2+ (range 3.0-6.0 except if only 1 patient in category), 8.0 in FAST4+/MDS2- (range 6.5-9.0), and 8.0 in FAST4+/MDS2+ patients (range 6.5-10.0), similar at both sites.
Conclusion: Median 0-10 PATGL was 2-3-fold higher in FAST4+/MDS2+ patients than in FAST4-/MDS2-. Similar patterns were seen with most diagnoses other than for FM in primary FM, higher in non-inflammatory than in inflammatory diagnoses. These patterns were similar patterns at 2 independent sites on 2 continents. Differences according to FAST4 and MDS2 to screen for FM and DEP were considerably greater than according to any primary diagnosis, feasibly recognized on a single MDHAQ.
 Table: Median patient global assessment in 928 patients with various rheumatic diagnoses at 2 sites, according to 2 feasibly collected and scored indices within a multidimensional health assessment questionnaire (MDHAQ), fibromyalgia assessment screening tool (FAST4) and MDHAQ depression screen (MDS2)
Table: Median patient global assessment in 928 patients with various rheumatic diagnoses at 2 sites, according to 2 feasibly collected and scored indices within a multidimensional health assessment questionnaire (MDHAQ), fibromyalgia assessment screening tool (FAST4) and MDHAQ depression screen (MDS2)*=Only 1 patient in category, NA=No patient in category
Disclosures: T. Pincus, Medical History Services, LLC, Medical History Services LLC; K. Gibson, Eli Lilly Pty Ltd; J. Schmukler, None; T. Li, None; J. Block, None.

