Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1007: Metabolic Disorders and Abnormal Dietary Patterns and Their Association with Psoriatic Arthritis Activity: The Dietary Intervention in PsA (DIPSA) Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Lihi Eder, MD, PhD
University of Toronto
Toronto, ON, Canada
Abstract Poster Presenter(s)
Lihi Eder1, Charlene Compher2, Helen Emanoilidis3, Ryan F. Quinn2, Dafna Gladman4, Vinod Chandran5, Jose Scher6 and Alexis Ogdie7, 1Women’s College Research Institute, Department of Medicine, University of Toronto, Toronto, ON, Canada, 2University of Pennsylvania, Philadelphia, PA, 3Women's College Hospital, Toronto, ON, Canada, 4Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 5Departments of Medicine and Laboratory Medicine and Pathobiology, University of Toronto/ Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, Toronto, ON, Canada, 6New York University School of Medicine, New York, NY, 7Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA
Background/Purpose: Psoriatic Arthritis (PsA) is associated with obesity and its related metabolic abnormalities. The role of diet as an adjunct therapy in PsA remains unclear. We aimed to describe the prevalence of cardiometabolic abnormalities and adherence with healthy eating recommendations among patients participating in the DIPSA study and assess their association with measures of disease activity.
Methods: DIPSA is a randomized controlled trial (NCT04180904) that aims to compare the efficacy of Mediterranean diet and DASH-low caloric diet vs. standard of care as adjunct therapy in patients with PsA who are overweight or obese (BMI >25). Study participants must have a Disease Activity in Psoriatic Arthritis (DAPSA) score >10 and be on stable therapy. Baseline information on the first 32 patients enrolled in the study (out of expected 90 patients) was analyzed. The presence of cardiometabolic abnormalities was assessed based on medical history, physical examination and laboratory tests. All participants completed a 24-h dietary recall of foods/beverages consumed during 3 separate weekdays. Healthy Eating Index (HEI) 2015, a diet quality score which evaluates adherence to dietary guidelines for Americans, was calculated. The score includes 13 food components (9 components encouraged, 4 to limit) with a range of 0 (low) to 5 or 10 (high). We describe the baseline cardiometabolic abnormalities and adherence with healthy diet scores and assess their correlation with PsA disease activity measures.
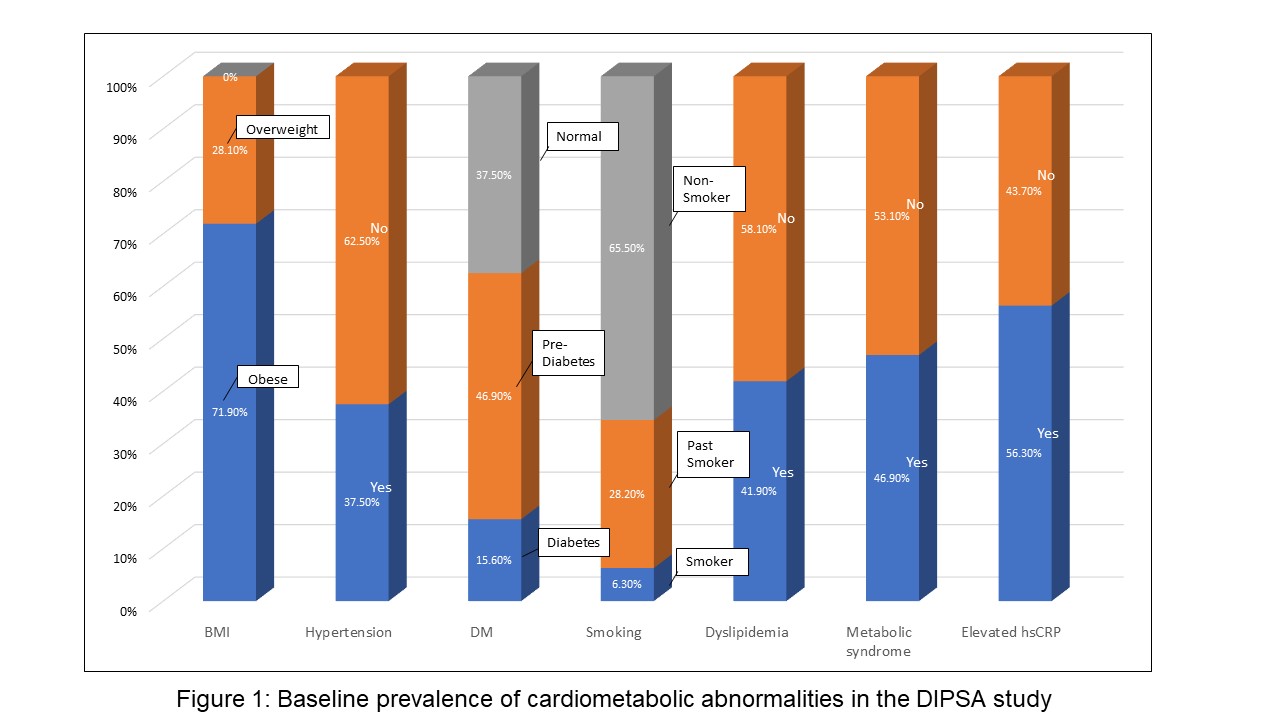
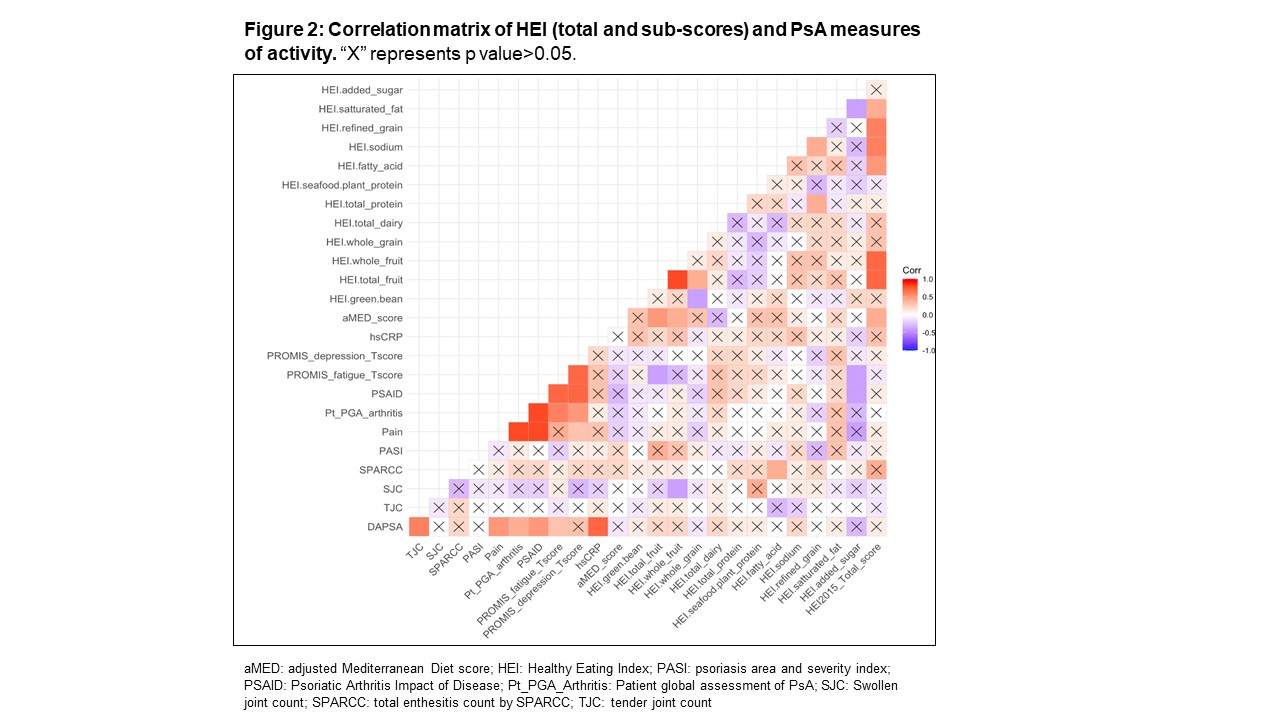
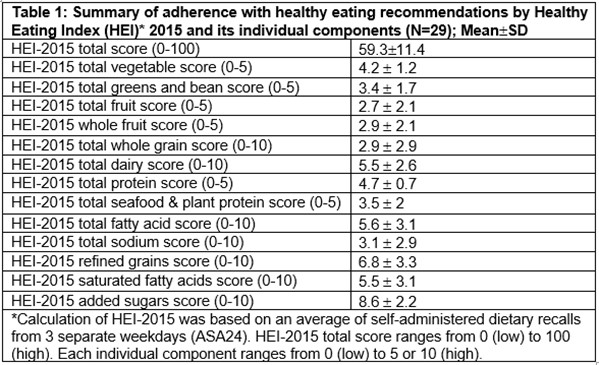
Results: The mean age of study participants was 53.3 years (71.9% females). The mean DAPSA, tender and swollen joint counts were 23.6, 6.6 and 1.1, respectively. A significant proportion of the patients had obesity (71.9%) and related metabolic abnormalities, such as dyslipidemia (41.9%), hypertension (37.5%), metabolic syndrome (46.9%) (Figure 1). Mean HEI-2015 was 59.3 with higher adherence scores (HEI-2015 >50; 25%Q1) found in females than males (91% vs. 57%, respectively, p< 0.05). No differences in HEI-2015 scores were found between age and level of education groups. Of the individual HEI-2015 food groups, the lowest adherence scores were found for whole grain and total sodium consumption (Table 1). Significant correlation was found between lower added sugar and both lower fatigue and lower PSAID scores (Figure 2), and correlation between greater whole fruit consumption and lower swollen joint count. Additionally, a correlation was found between the unsaturated than saturated fatty acids and higher enthesitis score.
Conclusion: High prevalence of metabolic abnormalities was found in patients with PsA starting a diet intervention study. Adherence with healthy diet eating recommendations at baseline was higher in female patients and individual foods, particularly sugar and whole fruit consumption, were associated with PsA measures of disease activity. The DIPSA study will determine the role of dietary interventions as adjunct therapy in PsA.
Disclosures: L. Eder, AbbVie, Lilly, Novartis, UCB, Pfizer; C. Compher, None; H. Emanoilidis, None; R. Quinn, None; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; V. Chandran, AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Amgen, Pfizer Inc, UCB; J. Scher, Janssen, Pfizer, AbbVie, Sanofi, Novartis, UCB; A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB.
Background/Purpose: Psoriatic Arthritis (PsA) is associated with obesity and its related metabolic abnormalities. The role of diet as an adjunct therapy in PsA remains unclear. We aimed to describe the prevalence of cardiometabolic abnormalities and adherence with healthy eating recommendations among patients participating in the DIPSA study and assess their association with measures of disease activity.
Methods: DIPSA is a randomized controlled trial (NCT04180904) that aims to compare the efficacy of Mediterranean diet and DASH-low caloric diet vs. standard of care as adjunct therapy in patients with PsA who are overweight or obese (BMI >25). Study participants must have a Disease Activity in Psoriatic Arthritis (DAPSA) score >10 and be on stable therapy. Baseline information on the first 32 patients enrolled in the study (out of expected 90 patients) was analyzed. The presence of cardiometabolic abnormalities was assessed based on medical history, physical examination and laboratory tests. All participants completed a 24-h dietary recall of foods/beverages consumed during 3 separate weekdays. Healthy Eating Index (HEI) 2015, a diet quality score which evaluates adherence to dietary guidelines for Americans, was calculated. The score includes 13 food components (9 components encouraged, 4 to limit) with a range of 0 (low) to 5 or 10 (high). We describe the baseline cardiometabolic abnormalities and adherence with healthy diet scores and assess their correlation with PsA disease activity measures.
Results: The mean age of study participants was 53.3 years (71.9% females). The mean DAPSA, tender and swollen joint counts were 23.6, 6.6 and 1.1, respectively. A significant proportion of the patients had obesity (71.9%) and related metabolic abnormalities, such as dyslipidemia (41.9%), hypertension (37.5%), metabolic syndrome (46.9%) (Figure 1). Mean HEI-2015 was 59.3 with higher adherence scores (HEI-2015 >50; 25%Q1) found in females than males (91% vs. 57%, respectively, p< 0.05). No differences in HEI-2015 scores were found between age and level of education groups. Of the individual HEI-2015 food groups, the lowest adherence scores were found for whole grain and total sodium consumption (Table 1). Significant correlation was found between lower added sugar and both lower fatigue and lower PSAID scores (Figure 2), and correlation between greater whole fruit consumption and lower swollen joint count. Additionally, a correlation was found between the unsaturated than saturated fatty acids and higher enthesitis score.
Conclusion: High prevalence of metabolic abnormalities was found in patients with PsA starting a diet intervention study. Adherence with healthy diet eating recommendations at baseline was higher in female patients and individual foods, particularly sugar and whole fruit consumption, were associated with PsA measures of disease activity. The DIPSA study will determine the role of dietary interventions as adjunct therapy in PsA.



Disclosures: L. Eder, AbbVie, Lilly, Novartis, UCB, Pfizer; C. Compher, None; H. Emanoilidis, None; R. Quinn, None; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; V. Chandran, AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Amgen, Pfizer Inc, UCB; J. Scher, Janssen, Pfizer, AbbVie, Sanofi, Novartis, UCB; A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB.

