Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1017: Minimal Important Difference (MID), Minimal Detectable Change (MDC), and Disease Activity Thresholds for Two Novel Composite Instruments (3VAS, 4VAS) in Patients with Psoriatic Arthritis: Pooled Analysis of Three Phase 3 Studies
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

William Tillett, MD, PhD
Royal National Hospital for rheumatic diseases
Bath, United Kingdom
Abstract Poster Presenter(s)
William Tillett1, Laura Coates2, Marijn Vis3, Joseph Merola4, Enrique R Soriano5, Michelle Perate6, May Shawi7, Miriam Zimmermann8, Emmanouil Rampakakis9, Mohamed Sharaf10, Peter Nash11 and Philip Helliwell12, 1Royal National Hospital for Rheumatic Diseases, Bath, United Kingdom, 2Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK, Oxford, England, United Kingdom, 3Erasmus MC Universitair Medisch Centrum, Rotterdam, Netherlands, 4Harvard Medical School, Brigham and Women's Hospital, Boston, MA, 5Rheumatology Unit and University Institute, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina, 6Janssen Scientific Affairs, LLC, Horsham, PA, 7Immunology Global Medical Affairs, Janssen Pharmaceutical Companies of Johnson & Johnson, Horsham, PA, 8Immunology, Janssen Scientific Affairs, LLC, Zug, Switzerland, 9McGill University, Department of Pediatrics and JSS Medical Research, Montréal, QC, Canada, 10Johnson & Johnson, Middle East FZ LLC, Dubai, United Arab Emirates, 11School of Medicine, Griffith University, Sunshine Coast, Australia, 12Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom
Background/Purpose: Though continuous composite measures of disease activity for psoriatic arthritis (PsA) assessment exist, abbreviated measures that are more feasible for screening in routine clinical practice are needed. The 3 Visual Analogue Scale (VAS) and 4 VAS scores were developed by abridging the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Composite Exercise (GRACE) measure to be the first short multidimensional composite measures specifically for use in PsA routine clinical care. The measures were shown to have superior performance than several established composite measures using small datasets,1. GRAPPA members recommended further testing of 3VAS/4VAS in observational and trial datasets.
Methods: This post-hoc analysis of the DISCOVER 1/2 and COSMOS studies2-4 used pooled data through week (W) 24 from all treatment groups across studies. The correlation of 3VAS/4VAS with DAPSA, PASDAS, physician global assessment (PhGA), and patient GA (PtGA) was assessed with Pearson's correlation coefficient. Minimal Important Difference (MID) was assessed with 4 distribution-based methods (based on standard error of the measurement, effect size, reliable change index [RCI], and RCIdiff). Minimal detectable change (MDC) was assessed with the standard formula (Table 2). Clinically relevant thresholds for low, moderate and high disease activity were estimated with receiver operating characteristic analysis and DAPSA (≤4, >4-≤14, >14-≤28, >28), PASDAS (≤1.9, >1.9-≤3.2, >3.2-< 5.4, ≥5.4), and PhGA/PtGA (≤1, >1-≤3, >3-≤6, >6 cm) as anchors.
Results: 1,405 pts were included, of whom 51.3% were male, with a mean (SD) age of 47.1 (11.8), and PsA duration of 6.4 (6.5) years. At BL, the mean (SD) 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA scores of 6.4 (1.6), 6.3 (1.6), 45.8 (20.2), 6.5 (1.1), 6.5 (1.6), and 6.7 (2.0), respectively, reflected high levels of disease activity. Through W24, both 3VAS and 4VAS showed very strong correlation with PtGA (r3VAS=0.92, r4VAS=0.94) and PASDAS (r3VAS=0.81, r4VAS=0.82), strong with PhGA (r3VAS=0.77, r4VAS=0.74), and moderate to strong with DAPSA (r3VAS=0.59, r4VAS=0.61). Calculated MIDs were 0.9 (range: 0.7-1.3 across methodologies) for 3VAS and 0.9 (range: 0.6-1.3) for 4VAS (Table 1). MDC estimates were 3.3 (range 2.1-4.2 across follow-up intervals) for 3VAS and 3.2 (range: 2-4) for 4VAS (Table 2). Cut-off values for low, moderate and high disease activity were 2.0, 3.4, and 4.9 for 3VAS and 2.1, 3.5 and 5.1 for 4VAS (Figure).
Conclusion: Using a large pooled clinical trial dataset of pts with active PsA, we have calculated clinically relevant thresholds for improvement, as well as disease activity thresholds, for 3VAS and 4VAS. These estimates are generally comparable to those previously reported5 and can be used to set treatment targets as well as screen disease activity in routine care when resources are limited or during remote pt monitoring.
ReferencesTillett W et al. J Rheum. 2021 Mar 1;jrheum.201675 Deodhar A et al. Lancet. 2020 Apr 4;395(10230):1115 Mease PJ et al. Lancet. 2020 Apr 4;395(10230):1126 Coates LC et al. Ann Rheum Dis. 2022;81(3):359 Tillett W et al. Ann Rheum Dis. 2021;80:135
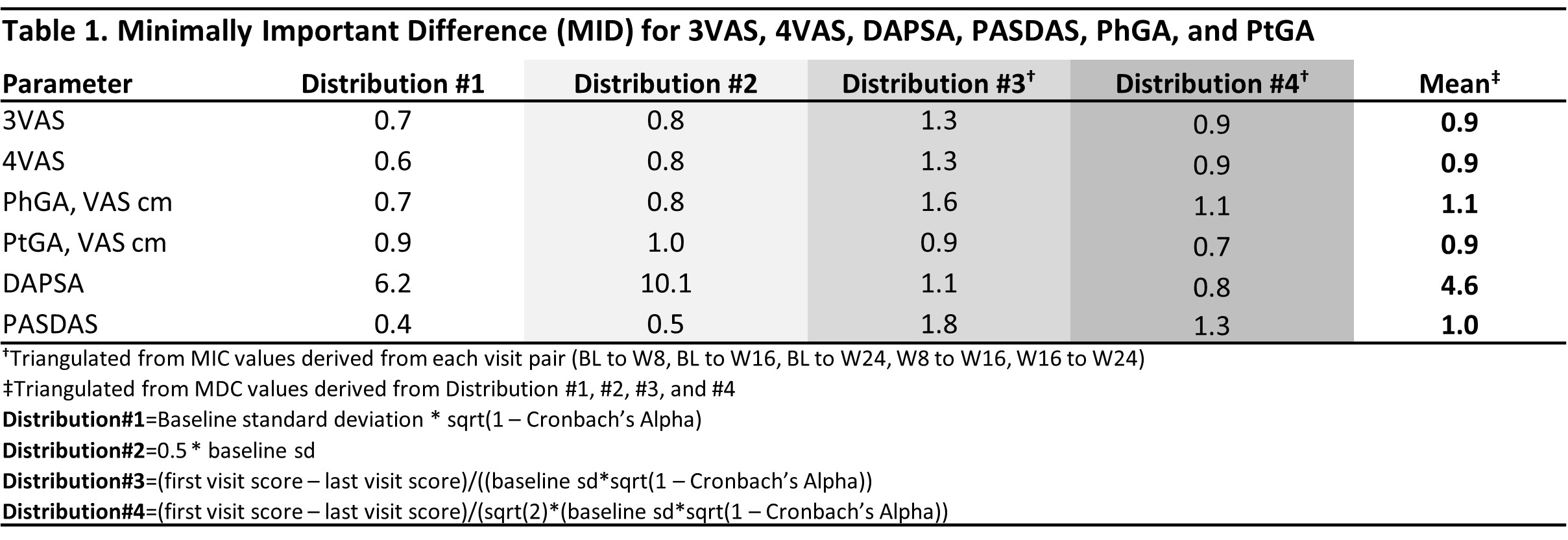 Table 1. Minimally Important Difference (MID) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA
Table 1. Minimally Important Difference (MID) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA
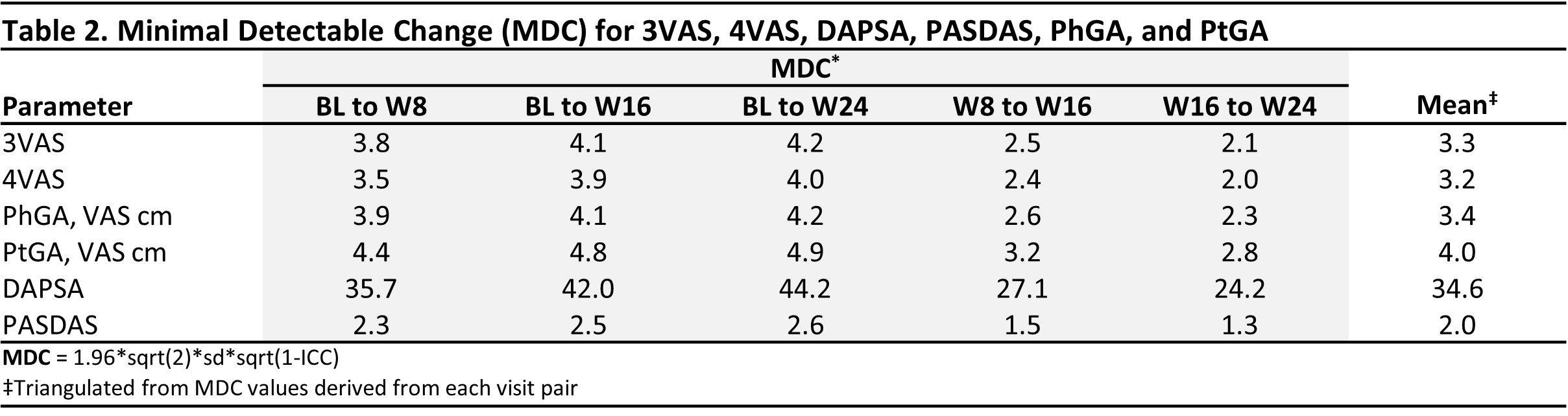 Table 2. Minimal Detectable Change (MDC) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA
Table 2. Minimal Detectable Change (MDC) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA
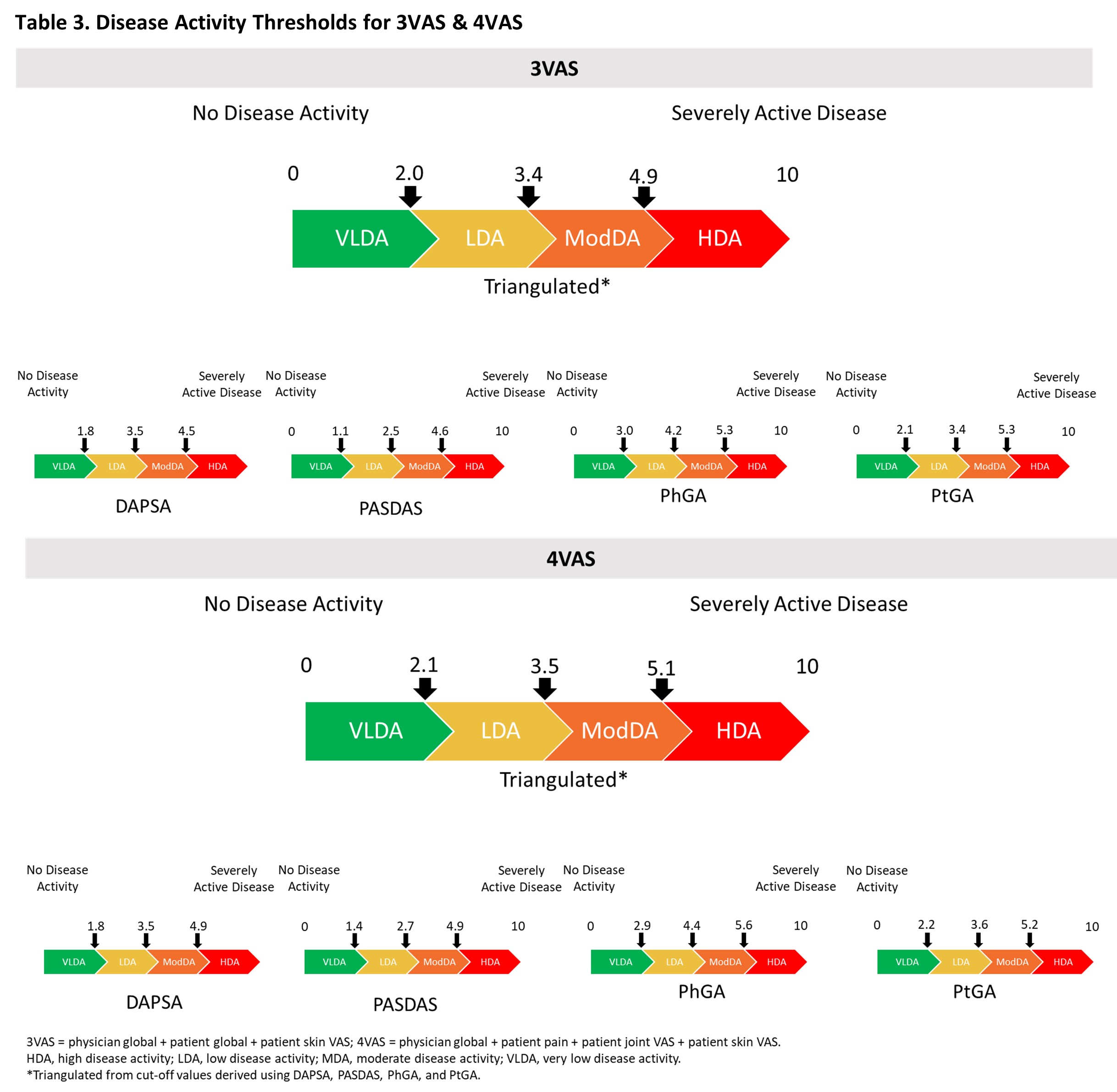 Table 3. Disease Activity Thresholds for 3VAS & 4VAS
Table 3. Disease Activity Thresholds for 3VAS & 4VAS
Disclosures: W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); M. Vis, AbbVie/Abbott, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, UCB, the Dutch Arthritis Foundation; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi; E. Soriano, AbbVie, Amgen, Bristol-Myers Squibb(BMS), Eli Lilly, Janssen, Novartis, Pfizer, UCB, Roche; M. Perate, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Zimmermann, Janssen Scientific Affairs, LLC, Johnson & Johnson; E. Rampakakis, Janssen, JSS Medical Research; M. Sharaf, Janssen Pharmaceutical Companies of Johnson and Johnson; P. Nash, AbbVie, Eli Lilly, Janssen, Gilead, Bristol-Myers Squibb (BMS), Celgene; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis.
Background/Purpose: Though continuous composite measures of disease activity for psoriatic arthritis (PsA) assessment exist, abbreviated measures that are more feasible for screening in routine clinical practice are needed. The 3 Visual Analogue Scale (VAS) and 4 VAS scores were developed by abridging the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Composite Exercise (GRACE) measure to be the first short multidimensional composite measures specifically for use in PsA routine clinical care. The measures were shown to have superior performance than several established composite measures using small datasets,1. GRAPPA members recommended further testing of 3VAS/4VAS in observational and trial datasets.
Methods: This post-hoc analysis of the DISCOVER 1/2 and COSMOS studies2-4 used pooled data through week (W) 24 from all treatment groups across studies. The correlation of 3VAS/4VAS with DAPSA, PASDAS, physician global assessment (PhGA), and patient GA (PtGA) was assessed with Pearson's correlation coefficient. Minimal Important Difference (MID) was assessed with 4 distribution-based methods (based on standard error of the measurement, effect size, reliable change index [RCI], and RCIdiff). Minimal detectable change (MDC) was assessed with the standard formula (Table 2). Clinically relevant thresholds for low, moderate and high disease activity were estimated with receiver operating characteristic analysis and DAPSA (≤4, >4-≤14, >14-≤28, >28), PASDAS (≤1.9, >1.9-≤3.2, >3.2-< 5.4, ≥5.4), and PhGA/PtGA (≤1, >1-≤3, >3-≤6, >6 cm) as anchors.
Results: 1,405 pts were included, of whom 51.3% were male, with a mean (SD) age of 47.1 (11.8), and PsA duration of 6.4 (6.5) years. At BL, the mean (SD) 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA scores of 6.4 (1.6), 6.3 (1.6), 45.8 (20.2), 6.5 (1.1), 6.5 (1.6), and 6.7 (2.0), respectively, reflected high levels of disease activity. Through W24, both 3VAS and 4VAS showed very strong correlation with PtGA (r3VAS=0.92, r4VAS=0.94) and PASDAS (r3VAS=0.81, r4VAS=0.82), strong with PhGA (r3VAS=0.77, r4VAS=0.74), and moderate to strong with DAPSA (r3VAS=0.59, r4VAS=0.61). Calculated MIDs were 0.9 (range: 0.7-1.3 across methodologies) for 3VAS and 0.9 (range: 0.6-1.3) for 4VAS (Table 1). MDC estimates were 3.3 (range 2.1-4.2 across follow-up intervals) for 3VAS and 3.2 (range: 2-4) for 4VAS (Table 2). Cut-off values for low, moderate and high disease activity were 2.0, 3.4, and 4.9 for 3VAS and 2.1, 3.5 and 5.1 for 4VAS (Figure).
Conclusion: Using a large pooled clinical trial dataset of pts with active PsA, we have calculated clinically relevant thresholds for improvement, as well as disease activity thresholds, for 3VAS and 4VAS. These estimates are generally comparable to those previously reported5 and can be used to set treatment targets as well as screen disease activity in routine care when resources are limited or during remote pt monitoring.
References
 Table 1. Minimally Important Difference (MID) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA
Table 1. Minimally Important Difference (MID) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA Table 2. Minimal Detectable Change (MDC) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA
Table 2. Minimal Detectable Change (MDC) for 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA Table 3. Disease Activity Thresholds for 3VAS & 4VAS
Table 3. Disease Activity Thresholds for 3VAS & 4VASDisclosures: W. Tillett, AbbVie, Amgen, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK); M. Vis, AbbVie/Abbott, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, UCB, the Dutch Arthritis Foundation; J. Merola, AbbVie, Biogen, BMS, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, UCB Pharma, Arena, Avotres, EMD, LEO Pharma, Merck, Regeneron, Sanofi; E. Soriano, AbbVie, Amgen, Bristol-Myers Squibb(BMS), Eli Lilly, Janssen, Novartis, Pfizer, UCB, Roche; M. Perate, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Shawi, Janssen Pharmaceutical Companies of Johnson and Johnson; M. Zimmermann, Janssen Scientific Affairs, LLC, Johnson & Johnson; E. Rampakakis, Janssen, JSS Medical Research; M. Sharaf, Janssen Pharmaceutical Companies of Johnson and Johnson; P. Nash, AbbVie, Eli Lilly, Janssen, Gilead, Bristol-Myers Squibb (BMS), Celgene; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis.

