Back
Poster Session B
Reproductive health
Session: (0939–0969) Reproductive Issues in Rheumatic Disorders Poster
0968: Neuropsychological Outcome of Children Born to Women with Systemic Sclerosis: Assessment Through a Self-administered Multidisciplinary Questionnaire in a Monocentric Cohort
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- EP
Eleonora Pedretti, MD
Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia
Brescia, Brescia, Italy
Abstract Poster Presenter(s)
Eleonora Pedretti1, Laura Andreoli1, Liala Moschetti1, Cecilia Nalli1, Anna Molinaro2, Jessica Galli2, Elisa Fazzi2, Franco Franceschini1, Angela Tincani3, Paolo Airò4 and Maria Grazia Lazzaroni1, 1Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia, Brescia, Italy, 2Unit of Child and Adolescent Neuropsychiatry, ASST Spedali Civili and University of Brescia, Brescia, Italy, 3Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia, Gussago, Italy, 4Rheumatology and Clinical Immunology Unit, ASST Spedali Civili, Brescia, Italy
Background/Purpose: In the last years, the rate of successful pregnancies has significantly increased in Systemic Sclerosis (SSc)1 women. However, the long-term outcome of their children remains an unexplored issue. Our aim is to evaluate the neuropsychiatric (NP) outcome of children born to SSc mothers.
Methods: An ad-hoc questionnaire, regarding different aspects of child's neurodevelopment (ND) (3 sections: childhood [0-5 years]; scholar age [6-11 years]; adolescence [12-18 years]), was created and administered to female SSc patients (ACR/EULAR 2013 criteria) attending our outpatient clinic during 2021 and who had at least 1 child. Children's NP characteristics were compared between 3 subgroups: A) born >10 years before SSc diagnosis; B) born £10 years before SSc diagnosis; C) born after SSc diagnosis. Results below are expressed as number/total number of answers collected for each question.
Results: 100 SSc women reported 189 pregnancies: 152 resulted in 154 live births (2 twin pregnancies, M/F=85/69). Deliveries occurred at a median of 39 (36-40) weeks of gestation: 28% of preterm births and 6% of severe preterm births (< 34 weeks of gestation). At least one NP alteration was reported in 42/119 (35%) subjects, more frequently in group B (57%), as compared to group A (32%, p:0.07) and group C (28%, p:0.09) (Figure 1). Sleep irregularities were the most frequently reported disorder (n=15). Comparisons between the 3 subgroups for each NP outcome are shown in Table 1: overall, a higher rate of NP alterations were reported in children of group B. A higher rate (12%) of severe preterm birth was observed in babies born in group C. In addition, SSc mothers declared that 7/123 (6%) children underwent a NP evaluation leading to a diagnosis in 3 cases: 1 cognitive delay, 1 learning disability (LD) and 1 autism spectrum disorder (ASD). These children were born 1 and 5 years before and 3 years after SSc diagnosis, respectively.
Conclusion: Children born to SSc mothers had a frequency of major NP alterations (cognitive delay, LD, ASD) similar to general pediatric population2. A higher frequency of minor ND disorders, especially sleep irregularities, was observed in children within 10 years before maternal diagnosis, possibly suggesting an impact of maternal chronic disease on the relationship with child in the first years of life. To confirm these self-reported preliminary data, the extension of the study will consist in a NP specialistic evaluation proposed to the offspring of SSc mothers aged £18 years.
GILS (Gruppo Italiano Lotta Sclerodermia) is kindly acknowledged for supporting the study with a grant.
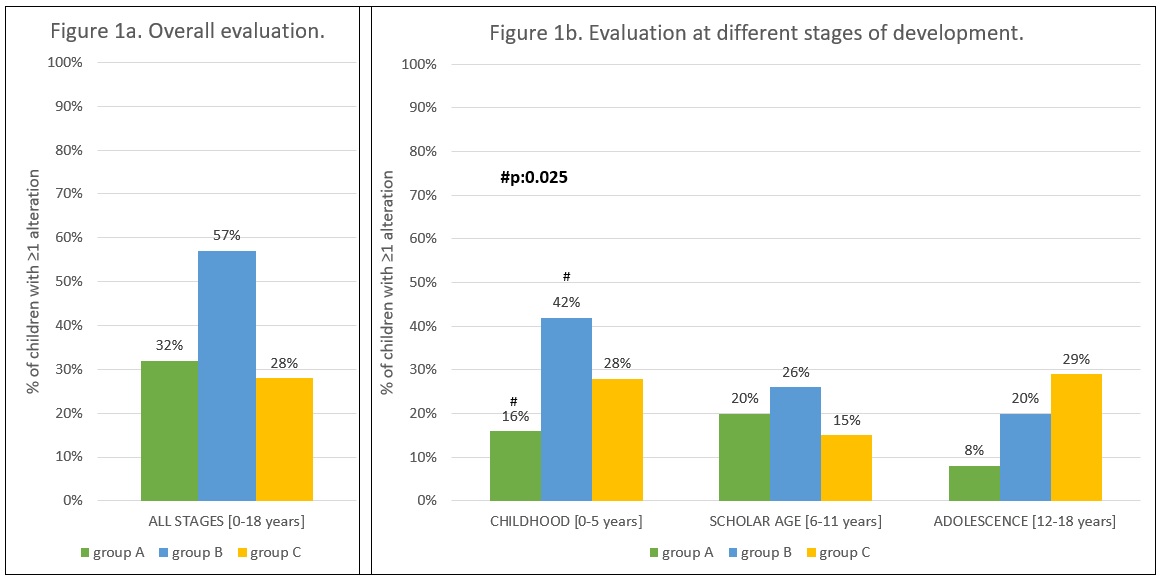 Figure 1. Frequency of children with at least one NP alteration according to the 3 age subgroups.
Figure 1. Frequency of children with at least one NP alteration according to the 3 age subgroups.
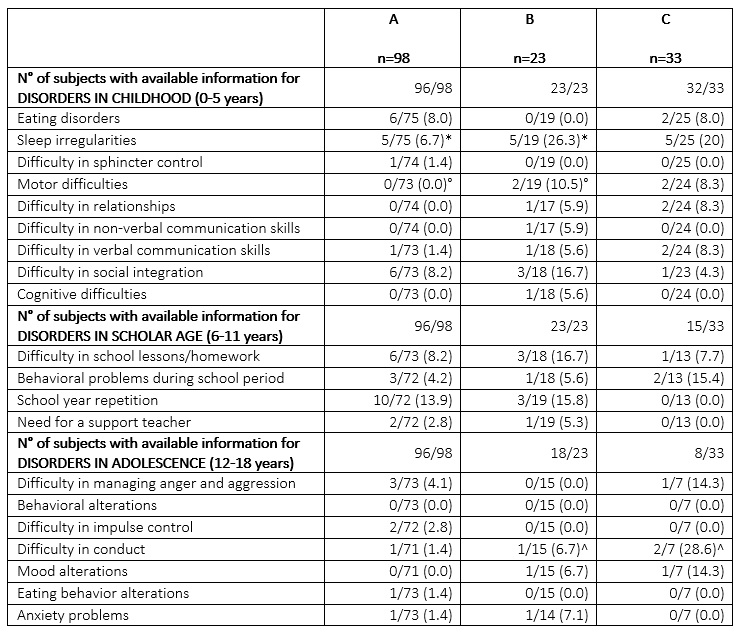 Table 1. Comparison of NP characteristics of the 154 children according to age subgroups: univariate analysis.
Table 1. Comparison of NP characteristics of the 154 children according to age subgroups: univariate analysis.
Results are presented as number/total number (%) of answers collected for each question. Variables were compared by Chi Squared/exact Fisher test. *p=0.03; °p=0.04; ^p=0.02.
Disclosures: E. Pedretti, None; L. Andreoli, GlaxoSmithKlein(GSK), Janssen, Novartis, UCB, Werfen; L. Moschetti, None; C. Nalli, None; A. Molinaro, None; J. Galli, None; E. Fazzi, None; F. Franceschini, None; A. Tincani, GlaxoSmithKlein(GSK), UCB; P. Airò, Bristol-Myers-Squibb, Boehringer Ingelheim, Roche, Jannsen, CSL Behring; M. Lazzaroni, None.
Background/Purpose: In the last years, the rate of successful pregnancies has significantly increased in Systemic Sclerosis (SSc)1 women. However, the long-term outcome of their children remains an unexplored issue. Our aim is to evaluate the neuropsychiatric (NP) outcome of children born to SSc mothers.
Methods: An ad-hoc questionnaire, regarding different aspects of child's neurodevelopment (ND) (3 sections: childhood [0-5 years]; scholar age [6-11 years]; adolescence [12-18 years]), was created and administered to female SSc patients (ACR/EULAR 2013 criteria) attending our outpatient clinic during 2021 and who had at least 1 child. Children's NP characteristics were compared between 3 subgroups: A) born >10 years before SSc diagnosis; B) born £10 years before SSc diagnosis; C) born after SSc diagnosis. Results below are expressed as number/total number of answers collected for each question.
Results: 100 SSc women reported 189 pregnancies: 152 resulted in 154 live births (2 twin pregnancies, M/F=85/69). Deliveries occurred at a median of 39 (36-40) weeks of gestation: 28% of preterm births and 6% of severe preterm births (< 34 weeks of gestation). At least one NP alteration was reported in 42/119 (35%) subjects, more frequently in group B (57%), as compared to group A (32%, p:0.07) and group C (28%, p:0.09) (Figure 1). Sleep irregularities were the most frequently reported disorder (n=15). Comparisons between the 3 subgroups for each NP outcome are shown in Table 1: overall, a higher rate of NP alterations were reported in children of group B. A higher rate (12%) of severe preterm birth was observed in babies born in group C. In addition, SSc mothers declared that 7/123 (6%) children underwent a NP evaluation leading to a diagnosis in 3 cases: 1 cognitive delay, 1 learning disability (LD) and 1 autism spectrum disorder (ASD). These children were born 1 and 5 years before and 3 years after SSc diagnosis, respectively.
Conclusion: Children born to SSc mothers had a frequency of major NP alterations (cognitive delay, LD, ASD) similar to general pediatric population2. A higher frequency of minor ND disorders, especially sleep irregularities, was observed in children within 10 years before maternal diagnosis, possibly suggesting an impact of maternal chronic disease on the relationship with child in the first years of life. To confirm these self-reported preliminary data, the extension of the study will consist in a NP specialistic evaluation proposed to the offspring of SSc mothers aged £18 years.
GILS (Gruppo Italiano Lotta Sclerodermia) is kindly acknowledged for supporting the study with a grant.
 Figure 1. Frequency of children with at least one NP alteration according to the 3 age subgroups.
Figure 1. Frequency of children with at least one NP alteration according to the 3 age subgroups. Table 1. Comparison of NP characteristics of the 154 children according to age subgroups: univariate analysis.
Table 1. Comparison of NP characteristics of the 154 children according to age subgroups: univariate analysis.Results are presented as number/total number (%) of answers collected for each question. Variables were compared by Chi Squared/exact Fisher test. *p=0.03; °p=0.04; ^p=0.02.
Disclosures: E. Pedretti, None; L. Andreoli, GlaxoSmithKlein(GSK), Janssen, Novartis, UCB, Werfen; L. Moschetti, None; C. Nalli, None; A. Molinaro, None; J. Galli, None; E. Fazzi, None; F. Franceschini, None; A. Tincani, GlaxoSmithKlein(GSK), UCB; P. Airò, Bristol-Myers-Squibb, Boehringer Ingelheim, Roche, Jannsen, CSL Behring; M. Lazzaroni, None.

