Back
Poster Session B
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: (0850–0880) Pediatric Rheumatology – Clinical Poster I: JIA
0850: Outcomes of Patients with Juvenile Idiopathic Arthritis Following Failure of Initial Tumor Necrosis Factor Inhibitor Medication in the Childhood Arthritis and Rheumatology Research Alliance Registry
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- MM
Melissa Mannion, MD
University of Alabama at Birmingham
Birmingham, AL, United States
Abstract Poster Presenter(s)
Melissa Mannion1, Shahla Amin2, Stephen Balevic3, Colleen Correll4, Timothy Beukelman1 and , for the CARRA Registry Investigators5, 1University of Alabama at Birmingham, Birmingham, AL, 2Childhood Arthritis and Rheumatology Research Alliance, Washington, DC, 3Duke University, Durham, NC, 4University of Minnesota, Minneapolis, MN, 5CARRA, Washington, DC
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) are the most commonly used first biologics to treat juvenile idiopathic arthritis (JIA), but it is unknown what subsequent biologic medications are most effective after failure of an initial TNFi. The objective of this study is to compare the effectiveness of using a second TNFi versus a non-TNFi following failure of a first TNFi in routine clinical practice.
Methods: We included individuals with a primary diagnosis of non-systemic polyarticular course JIA who received a TNFi as their first biologic medication and started a second biologic medication (index date) within 90 days of stopping the first TNFi on or after enrollment in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. Included patients had a completed 6 month follow up visit (+/- 3 months) after the index date and prior to February 29, 2020. Patients were excluded if they had active uveitis on the index date or if there was no Registry visit within 4 weeks of the index date. The primary outcome at the 6 month visit was inactive disease (ID) and minimal disease activity (MiDA) defined by the clinical juvenile arthritis disease activity score (cJADAS; cJADAS ID < 2.5 and cJADAS MiDA < 5). Missing data was accounted for by fully conditional specification multiple imputation. Propensity scores (PS) for likelihood of being in the second TNFi group were calculated by logistic regression with covariates from index date including active and limited joint count, physician and parent global assessment, childhood health assessment questionnaire, pain, erythrocyte sedimentation rate, time from diagnosis to index date, time from diagnosis to start of 1st TNFi, sex, JIA category, methotrexate use, glucocorticoid use, cJADAS, first TNFi, index date calendar year, history of uveitis. We used logistic regression to compare outcomes at the 6 month visit after switch to a second TNFi versus non-TNFi unadjusted and adjusted for PS quintile.
Results: Among 216 individuals with polyarticular course JIA included in the study, most received etanercept (84%) as first biologic and most stopped first TNFi for ineffectiveness (74%). 183 (85%) started a second TNFi and 33 (15%) started a non-TNFi. Adalimumab was the most common 2nd biologic (71% overall, 84% of 2nd TNFi) and tocilizumab was the most common non-TNFi 2nd biologic (9% overall, 58% of non-TNFi). On the index date, 56% of patients had used MTX (55% 2nd TNFi and 58% non-TNFi) and 12% of patients used glucocorticoids (11% 2nd TNFi and 15% non-TNFi). There was no difference in meeting cJADAS ID or MiDA criteria by treatment group even after adjusting for PS quintile.
Conclusion: Among polyarticular course JIA patients in a large North American registry following failure of a 1st TNFi, switch to a 2nd TNFi was more common than switch to a non-TNFi. There were no differences between those starting a 2nd TNFi or non-TNFi in achieving cJADAS ID or MiDA after 6 months. More research is necessary to determine which patients would benefit from change in treatment mechanism.
.jpg) Table 1: Characteristics of patients with polyarticular course JIA starting a second biologic (2nd TNFi or non-TNFi) following failure of an initial TNFi. (Abbreviations: TNFi – tumor necrosis factor inhibitor, IQR – interquartile range, Oligo – oligoarticular, RF – rheumatoid factor, poly – polyarticular, AJC – active joint count, LJC – limited joint count, CHAQ – childhood health assessment questionnaire, PGA – physician global assessment, PtGE – patient/parent global assessment of wellbeing, cJADAS – clinical juvenile arthritis disease activity score, MTX – methotrexate)
Table 1: Characteristics of patients with polyarticular course JIA starting a second biologic (2nd TNFi or non-TNFi) following failure of an initial TNFi. (Abbreviations: TNFi – tumor necrosis factor inhibitor, IQR – interquartile range, Oligo – oligoarticular, RF – rheumatoid factor, poly – polyarticular, AJC – active joint count, LJC – limited joint count, CHAQ – childhood health assessment questionnaire, PGA – physician global assessment, PtGE – patient/parent global assessment of wellbeing, cJADAS – clinical juvenile arthritis disease activity score, MTX – methotrexate)
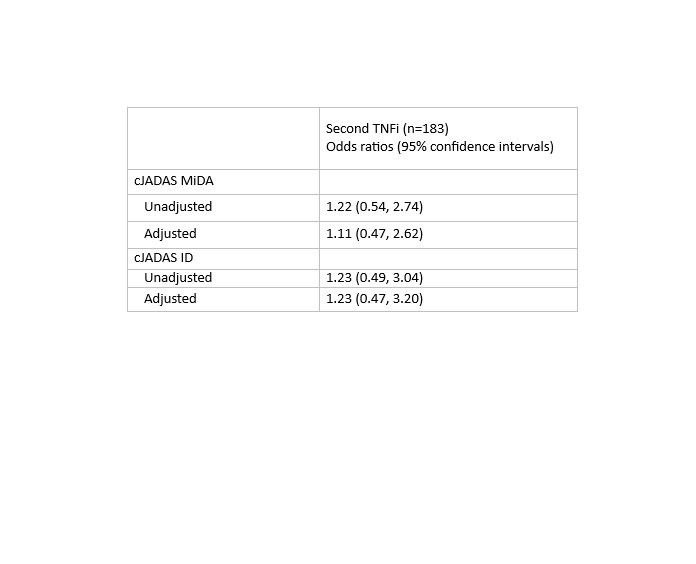 Table 2: Logistic regression estimates for meeting cJADAS ID or cJADAS MiDA at 6-months for patients with polyarticular course JIA following start of a 2nd biologic, referent group are those who started a non-TNFi. Models are adjusted for PS quintiles.
Table 2: Logistic regression estimates for meeting cJADAS ID or cJADAS MiDA at 6-months for patients with polyarticular course JIA following start of a 2nd biologic, referent group are those who started a non-TNFi. Models are adjusted for PS quintiles.
Disclosures: M. Mannion, None; S. Amin, None; S. Balevic, Purdue Pharma, UCB; C. Correll, None; T. Beukelman, Novartis, UCB; ,. for the CARRA Registry Investigators, None.
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) are the most commonly used first biologics to treat juvenile idiopathic arthritis (JIA), but it is unknown what subsequent biologic medications are most effective after failure of an initial TNFi. The objective of this study is to compare the effectiveness of using a second TNFi versus a non-TNFi following failure of a first TNFi in routine clinical practice.
Methods: We included individuals with a primary diagnosis of non-systemic polyarticular course JIA who received a TNFi as their first biologic medication and started a second biologic medication (index date) within 90 days of stopping the first TNFi on or after enrollment in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. Included patients had a completed 6 month follow up visit (+/- 3 months) after the index date and prior to February 29, 2020. Patients were excluded if they had active uveitis on the index date or if there was no Registry visit within 4 weeks of the index date. The primary outcome at the 6 month visit was inactive disease (ID) and minimal disease activity (MiDA) defined by the clinical juvenile arthritis disease activity score (cJADAS; cJADAS ID < 2.5 and cJADAS MiDA < 5). Missing data was accounted for by fully conditional specification multiple imputation. Propensity scores (PS) for likelihood of being in the second TNFi group were calculated by logistic regression with covariates from index date including active and limited joint count, physician and parent global assessment, childhood health assessment questionnaire, pain, erythrocyte sedimentation rate, time from diagnosis to index date, time from diagnosis to start of 1st TNFi, sex, JIA category, methotrexate use, glucocorticoid use, cJADAS, first TNFi, index date calendar year, history of uveitis. We used logistic regression to compare outcomes at the 6 month visit after switch to a second TNFi versus non-TNFi unadjusted and adjusted for PS quintile.
Results: Among 216 individuals with polyarticular course JIA included in the study, most received etanercept (84%) as first biologic and most stopped first TNFi for ineffectiveness (74%). 183 (85%) started a second TNFi and 33 (15%) started a non-TNFi. Adalimumab was the most common 2nd biologic (71% overall, 84% of 2nd TNFi) and tocilizumab was the most common non-TNFi 2nd biologic (9% overall, 58% of non-TNFi). On the index date, 56% of patients had used MTX (55% 2nd TNFi and 58% non-TNFi) and 12% of patients used glucocorticoids (11% 2nd TNFi and 15% non-TNFi). There was no difference in meeting cJADAS ID or MiDA criteria by treatment group even after adjusting for PS quintile.
Conclusion: Among polyarticular course JIA patients in a large North American registry following failure of a 1st TNFi, switch to a 2nd TNFi was more common than switch to a non-TNFi. There were no differences between those starting a 2nd TNFi or non-TNFi in achieving cJADAS ID or MiDA after 6 months. More research is necessary to determine which patients would benefit from change in treatment mechanism.
.jpg) Table 1: Characteristics of patients with polyarticular course JIA starting a second biologic (2nd TNFi or non-TNFi) following failure of an initial TNFi. (Abbreviations: TNFi – tumor necrosis factor inhibitor, IQR – interquartile range, Oligo – oligoarticular, RF – rheumatoid factor, poly – polyarticular, AJC – active joint count, LJC – limited joint count, CHAQ – childhood health assessment questionnaire, PGA – physician global assessment, PtGE – patient/parent global assessment of wellbeing, cJADAS – clinical juvenile arthritis disease activity score, MTX – methotrexate)
Table 1: Characteristics of patients with polyarticular course JIA starting a second biologic (2nd TNFi or non-TNFi) following failure of an initial TNFi. (Abbreviations: TNFi – tumor necrosis factor inhibitor, IQR – interquartile range, Oligo – oligoarticular, RF – rheumatoid factor, poly – polyarticular, AJC – active joint count, LJC – limited joint count, CHAQ – childhood health assessment questionnaire, PGA – physician global assessment, PtGE – patient/parent global assessment of wellbeing, cJADAS – clinical juvenile arthritis disease activity score, MTX – methotrexate) Table 2: Logistic regression estimates for meeting cJADAS ID or cJADAS MiDA at 6-months for patients with polyarticular course JIA following start of a 2nd biologic, referent group are those who started a non-TNFi. Models are adjusted for PS quintiles.
Table 2: Logistic regression estimates for meeting cJADAS ID or cJADAS MiDA at 6-months for patients with polyarticular course JIA following start of a 2nd biologic, referent group are those who started a non-TNFi. Models are adjusted for PS quintiles.Disclosures: M. Mannion, None; S. Amin, None; S. Balevic, Purdue Pharma, UCB; C. Correll, None; T. Beukelman, Novartis, UCB; ,. for the CARRA Registry Investigators, None.

