Back
Poster Session B
Osteoarthritis (OA) and related disorders
Session: (0833–0849) Orthopedics, Low Back Pain, and Rehabilitation Poster
0837: Providers’ Adoption of 2017 Guidelines on DMARD Use Perioperatively for Elective Total Hip and Knee Arthroplasty at a Single Academic Center
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CC
Christopher Ching, MD, BA
University of Utah
Salt Lake City, UT, United States
Abstract Poster Presenter(s)
Chris Ching1, Erica Mulcaire-Jones2, Andrew Shaffer1, Jeremy Gililland1, Michael Battistone3 and Dorota Lebiedz-Odrobina1, 1University of Utah, Salt Lake City, UT, 2Intermountain Healthcare, Salt Lake City, UT, 3Salt Lake City VA, Salt Lake City, UT
Background/Purpose: Rate of prosthetic joint infection (PJI) after total joint arthroplasty (TJA) is higher in patients with rheumatic conditions than in patients with OA. Our orthopedic group found an association between perioperative continuation of biologic medication and PJI in the veteran population (Carlson VR et al, J Arthroplasty 2021). The 2017 ACR/AAHKS guidelines make recommendations for the prescribing of conventional synthetic (cs), biologic (b) and targeted synthetic (ts) DMARDs around the time of elective TJA. The purpose of our study was to examine the adoption of guidelines by orthopedists and rheumatologists.
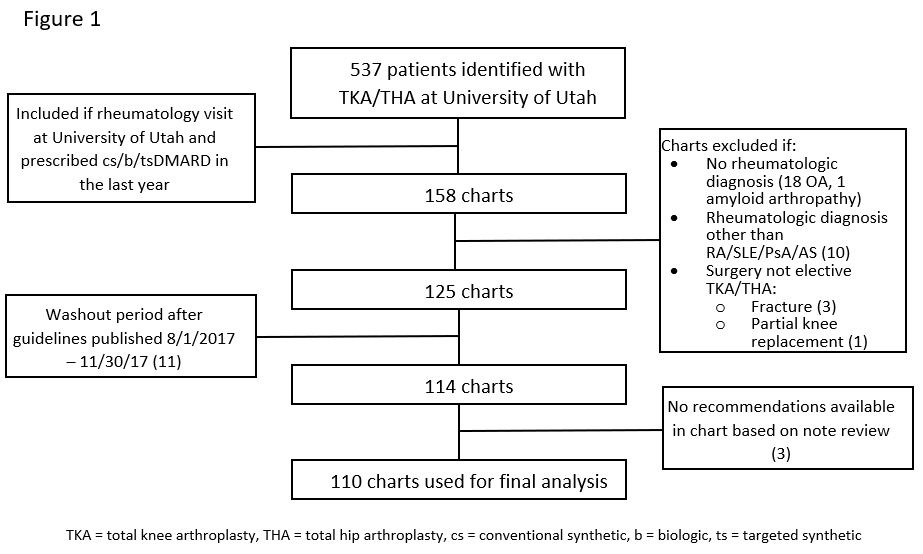
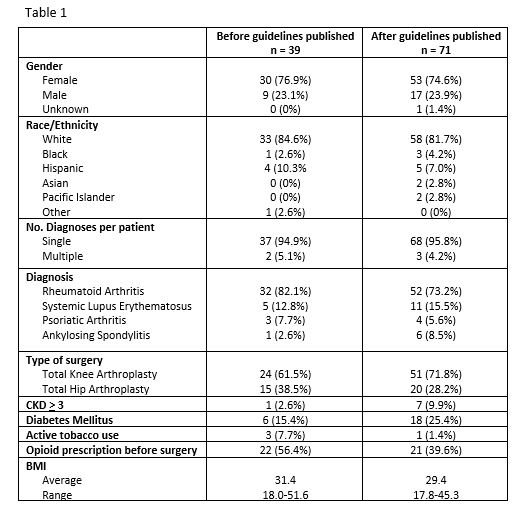
Methods: We performed a retrospective chart review of rheumatologic patients who had TJA between 10/2015 and 9/2019 at the University of Utah. 113 patients met our inclusion and exclusion criteria (Fig 1). Patient characteristics described in Table 1. Patients were analyzed in two groups, defined by whether they had been prescribed a csDMARD versus a b/tsDMARD in the year prior to TJA. Clinic notes were manually reviewed for documented recommendations by an orthopedist or rheumatologist regarding continuation or discontinuation of DMARD therapy perioperatively. We used the ACR/AAHKS 2017 perioperative guidelines to classify whether a provider made the correct recommendation. If b/tsDMARD was held longer than recommended by guidelines we classified as correct. If the correct recommendation was made but patient received medication inappropriately, we classified as correct. Chi-squared analysis determined whether a change occurred in rate of correct management of DMARDs after guideline publication in August 2017. Lastly, it was determined if patients had a prednisone prescription within 1 year prior to surgery date, and the mean dose before and after guideline publication was compared using Students T-test.
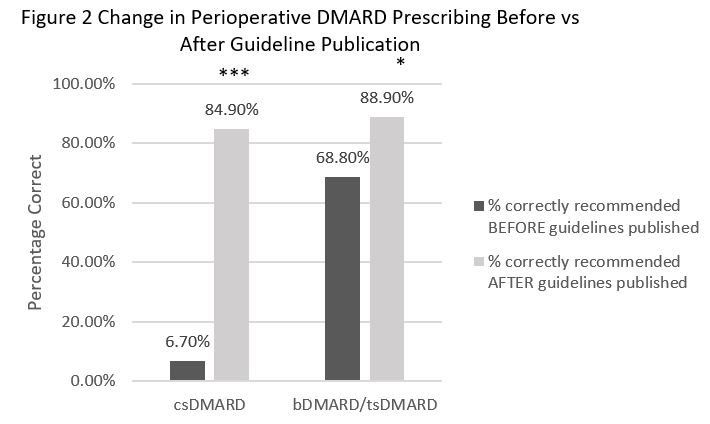
Results: There was a significant change in prescribing practices for csDMARDs and b/tsDMARDs before versus after guideline publication (fig 2). Two patients had the correct recommendations made by a provider but received an infusion prior to surgery. The average dose of prednisone prescribed before versus after guidelines released was 2 (range 0-12) mg and 0.7 (range 0-12) mg respectively (p=0.015). The number of PJIs in our cohort before and after guidelines were published was 0 and 2 respectively.
Conclusion: Our institution overall successfully adopted the 2017 ACR/AAHKS perioperative guidelines for TJA, but there is still room to improve. Our study was not powered to determine whether guidelines would lower PJI rate. Limitations include the involvement of an orthopedist at our institution in the guidelines, so guidelines may have been more widely adopted than at other institutions. Future directions include expanding our cohort to additional institutions to increase power for surgical complication rates, including PJI, before versus after guidelines published.
Disclosures: C. Ching, None; E. Mulcaire-Jones, None; A. Shaffer, None; J. Gililland, Stryker, OrthoGrid, DJO, MiCare Path; M. Battistone, None; D. Lebiedz-Odrobina, Pfizer.
Background/Purpose: Rate of prosthetic joint infection (PJI) after total joint arthroplasty (TJA) is higher in patients with rheumatic conditions than in patients with OA. Our orthopedic group found an association between perioperative continuation of biologic medication and PJI in the veteran population (Carlson VR et al, J Arthroplasty 2021). The 2017 ACR/AAHKS guidelines make recommendations for the prescribing of conventional synthetic (cs), biologic (b) and targeted synthetic (ts) DMARDs around the time of elective TJA. The purpose of our study was to examine the adoption of guidelines by orthopedists and rheumatologists.
Methods: We performed a retrospective chart review of rheumatologic patients who had TJA between 10/2015 and 9/2019 at the University of Utah. 113 patients met our inclusion and exclusion criteria (Fig 1). Patient characteristics described in Table 1. Patients were analyzed in two groups, defined by whether they had been prescribed a csDMARD versus a b/tsDMARD in the year prior to TJA. Clinic notes were manually reviewed for documented recommendations by an orthopedist or rheumatologist regarding continuation or discontinuation of DMARD therapy perioperatively. We used the ACR/AAHKS 2017 perioperative guidelines to classify whether a provider made the correct recommendation. If b/tsDMARD was held longer than recommended by guidelines we classified as correct. If the correct recommendation was made but patient received medication inappropriately, we classified as correct. Chi-squared analysis determined whether a change occurred in rate of correct management of DMARDs after guideline publication in August 2017. Lastly, it was determined if patients had a prednisone prescription within 1 year prior to surgery date, and the mean dose before and after guideline publication was compared using Students T-test.
Results: There was a significant change in prescribing practices for csDMARDs and b/tsDMARDs before versus after guideline publication (fig 2). Two patients had the correct recommendations made by a provider but received an infusion prior to surgery. The average dose of prednisone prescribed before versus after guidelines released was 2 (range 0-12) mg and 0.7 (range 0-12) mg respectively (p=0.015). The number of PJIs in our cohort before and after guidelines were published was 0 and 2 respectively.
Conclusion: Our institution overall successfully adopted the 2017 ACR/AAHKS perioperative guidelines for TJA, but there is still room to improve. Our study was not powered to determine whether guidelines would lower PJI rate. Limitations include the involvement of an orthopedist at our institution in the guidelines, so guidelines may have been more widely adopted than at other institutions. Future directions include expanding our cohort to additional institutions to increase power for surgical complication rates, including PJI, before versus after guidelines published.



Disclosures: C. Ching, None; E. Mulcaire-Jones, None; A. Shaffer, None; J. Gililland, Stryker, OrthoGrid, DJO, MiCare Path; M. Battistone, None; D. Lebiedz-Odrobina, Pfizer.

