Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0695–0723) Epidemiology and Public Health Poster I
0701: Reduced Humoral but Maintained Cellular Immune Response to a 3rd COVID-19 Vaccination in Patients with Immune-Mediated Inflammatory Diseases as Compared to Healthy Controls over a 3-months Observational Period
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- DM
Daniel Mrak, MD
Medical University of Vienna
Vienna, Austria
Abstract Poster Presenter(s)
Daniel Mrak1, Felix Kartnig1, Daniela Sieghart1, Elisabeth Simader1, Helga Radner1, Peter mandl2, Lisa Göschl1, Philipp Hofer3, Thomas Hummel1, Thomas Deimel4, Irina Gessl4, Renate Kain5, Stefan Winkler6, Josef Smolen4, Thomas Perkmann7, Helmuth Haslacher7, Daniel Aletaha8, Leonhard Heinz1 and Michael Bonelli1, 1Division of Rheumatology, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria, 2Division of Rheumatology, Department of Internal Medicine III, Medical University of Vienna, Wien, Austria, 3Department of Pathology, Medical University of Vienna, Vienna, Austria, 4Medical University of Vienna, Vienna, Austria, 5Department of Pathology, Medical University of Vienna, Vienna, 6Division of Infectious Diseases and Tropical Medicine, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria, 7Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria, 8Medical University Vienna, Wien, Austria
Background/Purpose: A third COVID-19 vaccination is currently recommended for patients under immunosuppression. However, a fast decline of antibodies against the SARS-CoV-2 receptor-binding domain (RBD) of the spike protein has been reported. Currently it remains unclear whether immunosuppressive therapy affects stability of a humoral and cellular immune response.
Methods: 51 patients with immune-mediated inflammatory diseases (IMID) under immunosuppression and 45 healthy controls (HCs) received a 3rd dose of an mRNA-based vaccination and were monitored over a 12-weeks period. Humoral immune response was assessed 4 and 12 weeks after 3rd dose. Antibodies were quantified using the Elecsys Anti-SARS-CoV-2 S immunoassay against the receptor-binding domain (RBD) of the spike protein. SARS-CoV-2-specific T cell responses were analyzed 1 and 12 weeks after 3rd dose and were quantified by IFN-γ ELISpot assays. Adverse events, including SARS-CoV-2 infections, were monitored over a 12-week period.
Results: At week 12 reduced anti-RBD antibody levels were observed in IMID patients as compared HCs (median antibody level 5400 BAU/ml [2074, 10400] versus 9500 BAU/ml [6610, 16300], p=0.003). Relative reduction in antibody levels was significantly higher in IMID patients as compared to HCs at week 12 (p=0.003). Lowest levels of anti-RBD antibody levels were detected in IMID patients who received a combination therapy with conventional synthetic and biological diseases modifying anti-rheumatic drugs. Number of SARS-CoV-2 specific T cells remained stable over 12 weeks in IMID patients and HCs. No serious adverse events were reported. Two IMID patients and two HCs were tested positive for SARS-CoV-2 between week 4 and 12.
Conclusion: A fast decline in anti-RBD antibodies was observed in IMID patients under immunosuppression. A 4th vaccination should therefore be considered in this vulnerable group of patients.
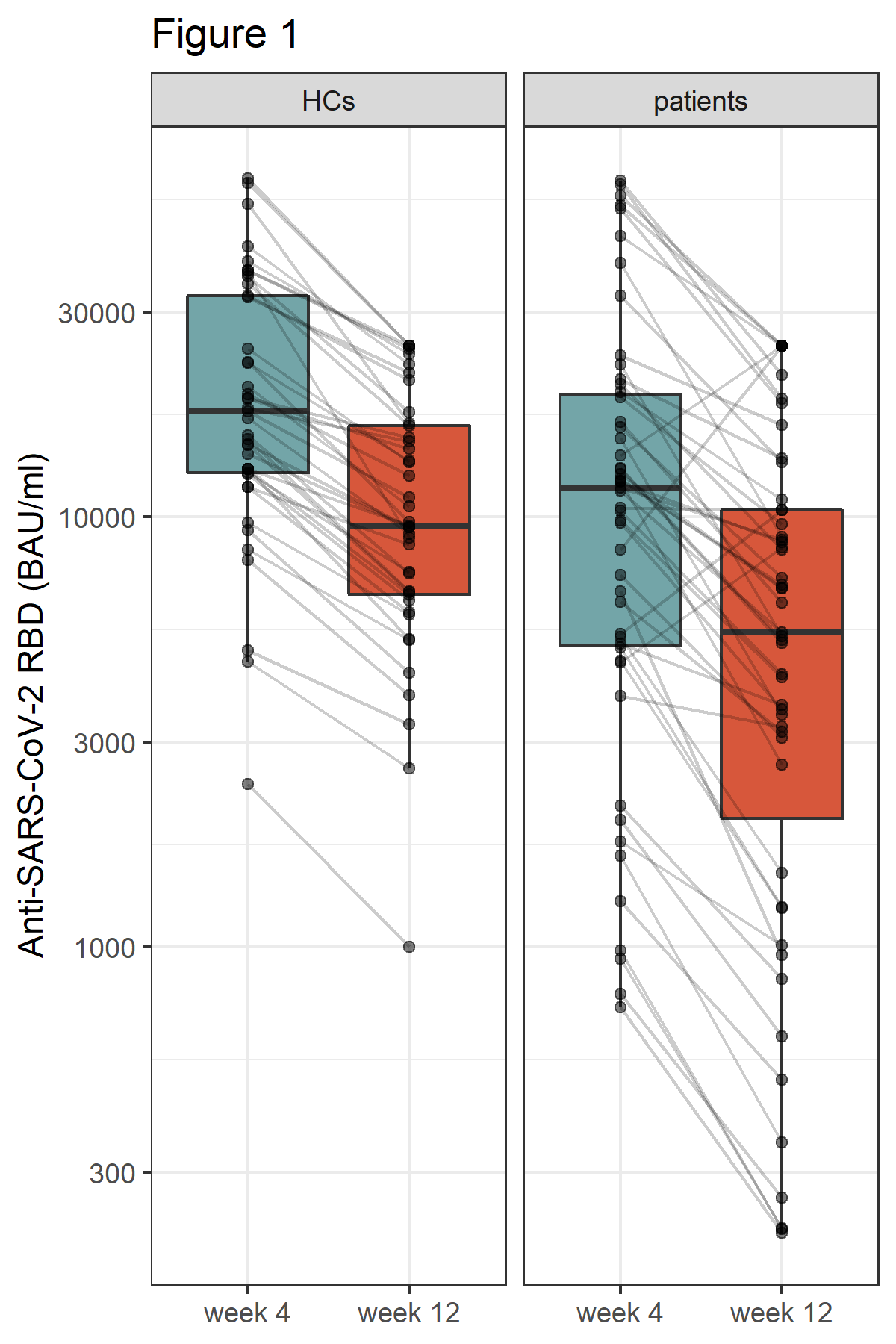 Figure 1: Humoral immune response 4 and 12 weeks after 3rd vaccine dose in healthy controls (HCs) and patients. Antibodies to the receptor-binding domain (RBD) of the viral spike (S) protein were determined using an anti-SARS-CoV-2 immunoassay.
Figure 1: Humoral immune response 4 and 12 weeks after 3rd vaccine dose in healthy controls (HCs) and patients. Antibodies to the receptor-binding domain (RBD) of the viral spike (S) protein were determined using an anti-SARS-CoV-2 immunoassay.
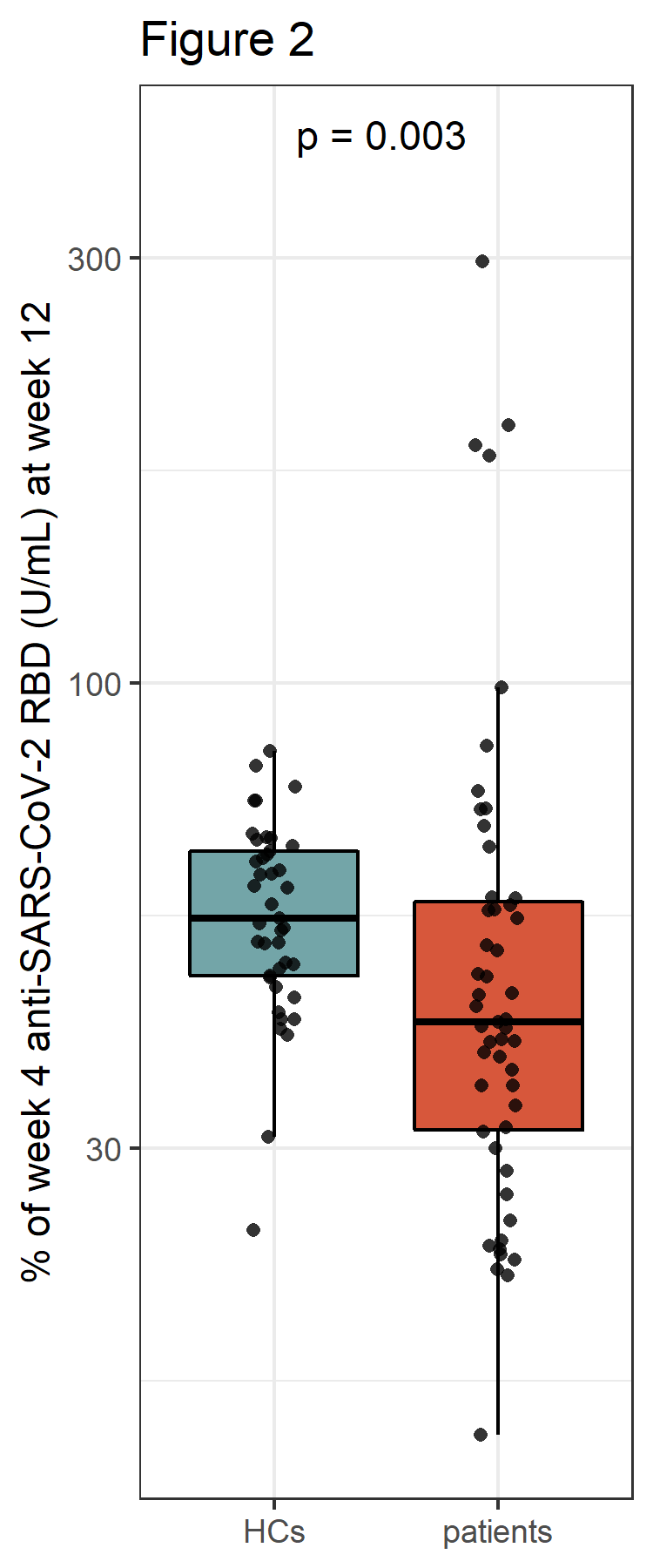 Figure 2: Relative change of anti-SARS-CoV-2 RBD antibodies between week 4 and week 12 after 3rd vaccine dose in healthy controls (HCs) as compared to patients
Figure 2: Relative change of anti-SARS-CoV-2 RBD antibodies between week 4 and week 12 after 3rd vaccine dose in healthy controls (HCs) as compared to patients
Disclosures: D. Mrak, Pfizer; F. Kartnig, Boehringer-Ingelheim, Eli Lilly; D. Sieghart, None; E. Simader, Boehringer-Ingelheim; H. Radner, None; P. mandl, AbbVie, Bristol-Myers Squibb(BMS), Celgene, Janssen, Eli Lilly, Novartis, Merck/MSD, Roche, UCB; L. Göschl, None; P. Hofer, None; T. Hummel, None; T. Deimel, None; I. Gessl, Eli Lilly, Boehringer-Ingelheim, Gilead; R. Kain, None; S. Winkler, None; J. Smolen, AbbVie, AstraZeneca, Eli Lilly, Novartis, Amgen, Bristol Myers Squibb, Galapagos-Gilead, Janssen, Merck-Sharp-Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, UCB; T. Perkmann, None; H. Haslacher, Glock Health, BlueSky Immunotherapies, Neutrolis; D. Aletaha, Novartis, SoBi, Sanofi, Amgen, Lilly, Merck, Pfizer, Roche, Sandoz, Janssen, AbbVie; L. Heinz, None; M. Bonelli, Eli Lilly.
Background/Purpose: A third COVID-19 vaccination is currently recommended for patients under immunosuppression. However, a fast decline of antibodies against the SARS-CoV-2 receptor-binding domain (RBD) of the spike protein has been reported. Currently it remains unclear whether immunosuppressive therapy affects stability of a humoral and cellular immune response.
Methods: 51 patients with immune-mediated inflammatory diseases (IMID) under immunosuppression and 45 healthy controls (HCs) received a 3rd dose of an mRNA-based vaccination and were monitored over a 12-weeks period. Humoral immune response was assessed 4 and 12 weeks after 3rd dose. Antibodies were quantified using the Elecsys Anti-SARS-CoV-2 S immunoassay against the receptor-binding domain (RBD) of the spike protein. SARS-CoV-2-specific T cell responses were analyzed 1 and 12 weeks after 3rd dose and were quantified by IFN-γ ELISpot assays. Adverse events, including SARS-CoV-2 infections, were monitored over a 12-week period.
Results: At week 12 reduced anti-RBD antibody levels were observed in IMID patients as compared HCs (median antibody level 5400 BAU/ml [2074, 10400] versus 9500 BAU/ml [6610, 16300], p=0.003). Relative reduction in antibody levels was significantly higher in IMID patients as compared to HCs at week 12 (p=0.003). Lowest levels of anti-RBD antibody levels were detected in IMID patients who received a combination therapy with conventional synthetic and biological diseases modifying anti-rheumatic drugs. Number of SARS-CoV-2 specific T cells remained stable over 12 weeks in IMID patients and HCs. No serious adverse events were reported. Two IMID patients and two HCs were tested positive for SARS-CoV-2 between week 4 and 12.
Conclusion: A fast decline in anti-RBD antibodies was observed in IMID patients under immunosuppression. A 4th vaccination should therefore be considered in this vulnerable group of patients.
 Figure 1: Humoral immune response 4 and 12 weeks after 3rd vaccine dose in healthy controls (HCs) and patients. Antibodies to the receptor-binding domain (RBD) of the viral spike (S) protein were determined using an anti-SARS-CoV-2 immunoassay.
Figure 1: Humoral immune response 4 and 12 weeks after 3rd vaccine dose in healthy controls (HCs) and patients. Antibodies to the receptor-binding domain (RBD) of the viral spike (S) protein were determined using an anti-SARS-CoV-2 immunoassay. Figure 2: Relative change of anti-SARS-CoV-2 RBD antibodies between week 4 and week 12 after 3rd vaccine dose in healthy controls (HCs) as compared to patients
Figure 2: Relative change of anti-SARS-CoV-2 RBD antibodies between week 4 and week 12 after 3rd vaccine dose in healthy controls (HCs) as compared to patientsDisclosures: D. Mrak, Pfizer; F. Kartnig, Boehringer-Ingelheim, Eli Lilly; D. Sieghart, None; E. Simader, Boehringer-Ingelheim; H. Radner, None; P. mandl, AbbVie, Bristol-Myers Squibb(BMS), Celgene, Janssen, Eli Lilly, Novartis, Merck/MSD, Roche, UCB; L. Göschl, None; P. Hofer, None; T. Hummel, None; T. Deimel, None; I. Gessl, Eli Lilly, Boehringer-Ingelheim, Gilead; R. Kain, None; S. Winkler, None; J. Smolen, AbbVie, AstraZeneca, Eli Lilly, Novartis, Amgen, Bristol Myers Squibb, Galapagos-Gilead, Janssen, Merck-Sharp-Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, UCB; T. Perkmann, None; H. Haslacher, Glock Health, BlueSky Immunotherapies, Neutrolis; D. Aletaha, Novartis, SoBi, Sanofi, Amgen, Lilly, Merck, Pfizer, Roche, Sandoz, Janssen, AbbVie; L. Heinz, None; M. Bonelli, Eli Lilly.

