Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0695–0723) Epidemiology and Public Health Poster I
0710: Risk Factors Associated with COVID-19 Breakthrough Infection Among Patients with Systemic Autoimmune Rheumatic Diseases: A Cohort Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Naomi Patel, MD
Massachusetts General Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Naomi Patel1, Xiaosong Wang2, Xiaoqing Fu3, Yumeko Kawano2, Claire Cook3, Kathleen Vanni2, Grace Qian2, Emily Banasiak2, Emily Kowalski2, yuqing zhang4, Jeffrey Sparks5 and Zachary Wallace3, 1Massachusetts General Hospital, Sale Creek, TN, 2Brigham and Women's Hospital, Boston, MA, 3Massachusetts General Hospital, Boston, MA, 4Massachusetts General Hospital, Quincy, MA, 5Brigham and Women's Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Some patients with rheumatic disease on DMARDs may be at increased risk of poor response to SARS-CoV-2 vaccines and thus breakthrough COVID-19 infections. We aimed to identify factors associated with breakthrough infection after receipt of the initial vaccine series among patients with systemic autoimmune rheumatic diseases (SARDs).
Methods: In this retrospective cohort study, we identified patients with SARDs being treated with DMARDs and/or glucocorticoids in a multi-center healthcare system who received at least two doses of either the mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) vaccines or one dose of the Johnson & Johnson-Janssen (J&J) vaccine. We used electronic health record data to identify patients with SARS-CoV-2 infection, defined as either a positive SARS-CoV-2 antigen or polymerase chain reaction test, following vaccination. We calculated total person-months of follow-up based on the time from the index date (date of J&J vaccine or second Moderna or Pfizer-BioNTech vaccine) until SARS-CoV-2 infection, death, or until December 15, 2021, when the Omicron variant became the local dominant strain. We estimated the association of clinical characteristics with the risk of breakthrough infection using unadjusted and multivariable Cox regression.
Results: Between December 11, 2020 and December 15, 2021, we identified 11,551 vaccinated patients with SARDs (75% female, mean age 60.1 years) (Table 1). The most common SARD categories were inflammatory arthritis (8058, 70%) and connective tissue disease (2027, 18%). The incidence rates (95% CI) of infection per 1000 person-months for specific DMARDs were: 1.53 (0.96, 2.09) for antimalarial monotherapy, 2.53 (1.92, 3.13) for TNF inhibitors, 2.87 (1.17, 4.57) for Janus kinase inhibitors, 3.14 (1.36, 4.92) for IL-6 inhibitors, 4.21 (2.14, 6.27) for mycophenolate, 5.10 (2.60, 7.60) for CTLA-4 Ig, and 8.28 (4.46, 12.11) for anti-CD20 monoclonal antibodies (Table 2; Figure). Compared to antimalarial monotherapy, use of B cell depletion within 12 months (aHR 4.97, 95% CI: 2.69, 9.18), IL-6 inhibitors (aHR 2.12, 95% CI: 1.08, 4.17), CTLA-4 inhibitors (aHR 3.45, 95% CI: 1.86, 6.40), mycophenolate mofetil or mycophenolic acid (aHR 2.27, 95% CI: 1.22, 4.21), or other csDMARD (aHR 2.25, 95% CI: 1.32, 3.83) was associated with higher risk of breakthrough infection (Table 2). Those who received the Moderna vaccine had a lower risk of breakthrough infection compared to those who received Pfizer-BioNTech (aHR 0.59, 95% CI: 0.44 to 0.80). There was no association of sex or specific rheumatic disease diagnosis with the risk of breakthrough infection.
Conclusion: Among patients with SARDs who received their initial SARS-CoV-2 vaccine series, specific conventional synthetic and biologic DMARDs were associated with increased risk of COVID-19 breakthrough infection. Additional studies are needed to further investigate the efficacy of the Moderna versus Pfizer-BioNTech vaccine in patients with SARDs. These results highlight the need for additional mitigation strategies in this vulnerable population.
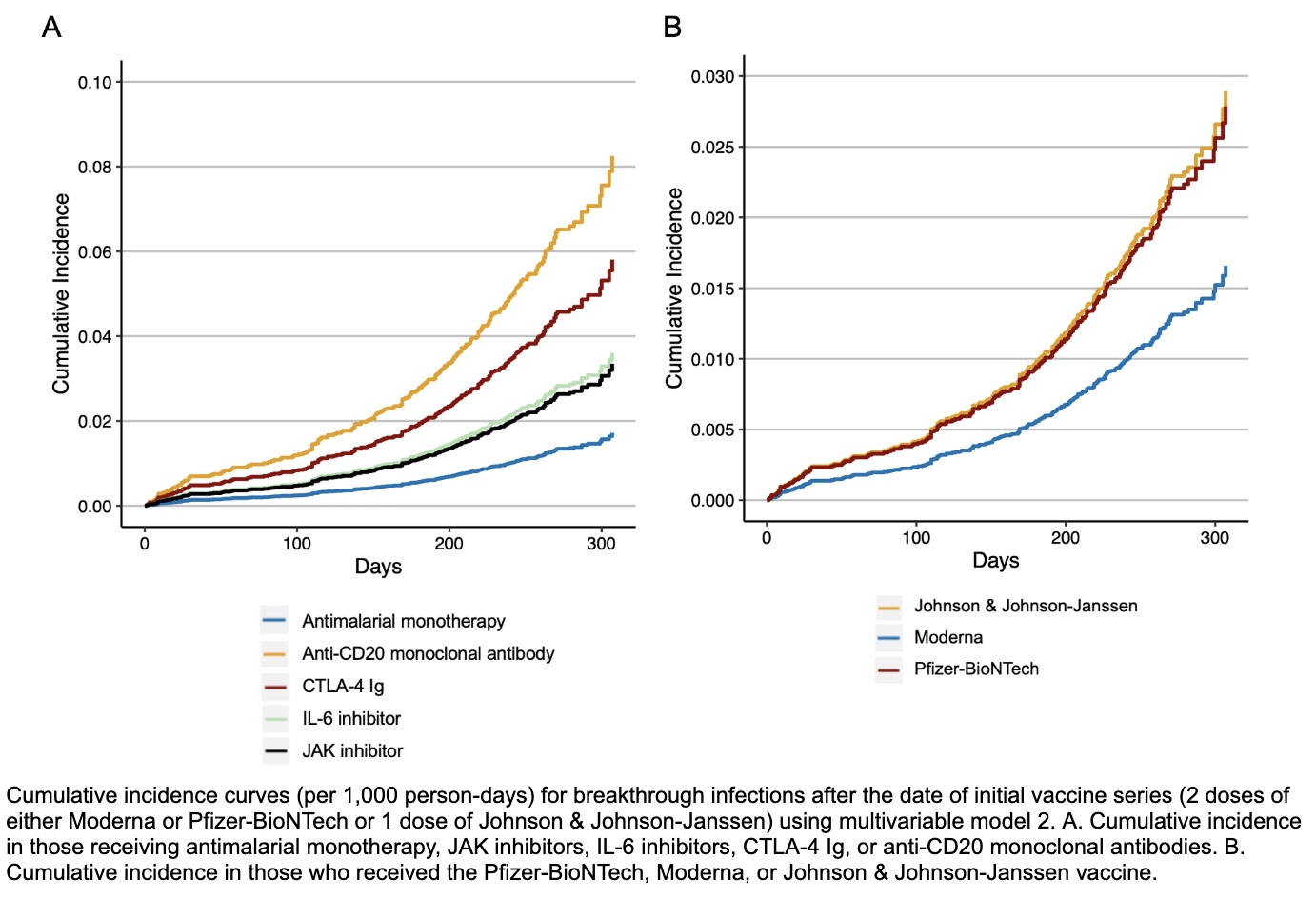 Figure. Cumulative incidence curves (per 1,000 person-days) demonstrating time to development of breakthrough infection after vaccination.
Figure. Cumulative incidence curves (per 1,000 person-days) demonstrating time to development of breakthrough infection after vaccination.
.jpg) Table 1. Baseline clinical characteristics at the time of completion of initial SARS-CoV-2 vaccine series of patients with systemic autoimmune rheumatic diseases.
Table 1. Baseline clinical characteristics at the time of completion of initial SARS-CoV-2 vaccine series of patients with systemic autoimmune rheumatic diseases.
.jpg) Table 2. Clinical characteristics at time of vaccination associated with breakthrough infection among patients with systemic autoimmune rheumatic diseases.
Table 2. Clinical characteristics at time of vaccination associated with breakthrough infection among patients with systemic autoimmune rheumatic diseases.
Disclosures: N. Patel, FVC Health; X. Wang, None; X. Fu, None; Y. Kawano, None; C. Cook, None; K. Vanni, None; G. Qian, None; E. Banasiak, None; E. Kowalski, None; y. zhang, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon.
Background/Purpose: Some patients with rheumatic disease on DMARDs may be at increased risk of poor response to SARS-CoV-2 vaccines and thus breakthrough COVID-19 infections. We aimed to identify factors associated with breakthrough infection after receipt of the initial vaccine series among patients with systemic autoimmune rheumatic diseases (SARDs).
Methods: In this retrospective cohort study, we identified patients with SARDs being treated with DMARDs and/or glucocorticoids in a multi-center healthcare system who received at least two doses of either the mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) vaccines or one dose of the Johnson & Johnson-Janssen (J&J) vaccine. We used electronic health record data to identify patients with SARS-CoV-2 infection, defined as either a positive SARS-CoV-2 antigen or polymerase chain reaction test, following vaccination. We calculated total person-months of follow-up based on the time from the index date (date of J&J vaccine or second Moderna or Pfizer-BioNTech vaccine) until SARS-CoV-2 infection, death, or until December 15, 2021, when the Omicron variant became the local dominant strain. We estimated the association of clinical characteristics with the risk of breakthrough infection using unadjusted and multivariable Cox regression.
Results: Between December 11, 2020 and December 15, 2021, we identified 11,551 vaccinated patients with SARDs (75% female, mean age 60.1 years) (Table 1). The most common SARD categories were inflammatory arthritis (8058, 70%) and connective tissue disease (2027, 18%). The incidence rates (95% CI) of infection per 1000 person-months for specific DMARDs were: 1.53 (0.96, 2.09) for antimalarial monotherapy, 2.53 (1.92, 3.13) for TNF inhibitors, 2.87 (1.17, 4.57) for Janus kinase inhibitors, 3.14 (1.36, 4.92) for IL-6 inhibitors, 4.21 (2.14, 6.27) for mycophenolate, 5.10 (2.60, 7.60) for CTLA-4 Ig, and 8.28 (4.46, 12.11) for anti-CD20 monoclonal antibodies (Table 2; Figure). Compared to antimalarial monotherapy, use of B cell depletion within 12 months (aHR 4.97, 95% CI: 2.69, 9.18), IL-6 inhibitors (aHR 2.12, 95% CI: 1.08, 4.17), CTLA-4 inhibitors (aHR 3.45, 95% CI: 1.86, 6.40), mycophenolate mofetil or mycophenolic acid (aHR 2.27, 95% CI: 1.22, 4.21), or other csDMARD (aHR 2.25, 95% CI: 1.32, 3.83) was associated with higher risk of breakthrough infection (Table 2). Those who received the Moderna vaccine had a lower risk of breakthrough infection compared to those who received Pfizer-BioNTech (aHR 0.59, 95% CI: 0.44 to 0.80). There was no association of sex or specific rheumatic disease diagnosis with the risk of breakthrough infection.
Conclusion: Among patients with SARDs who received their initial SARS-CoV-2 vaccine series, specific conventional synthetic and biologic DMARDs were associated with increased risk of COVID-19 breakthrough infection. Additional studies are needed to further investigate the efficacy of the Moderna versus Pfizer-BioNTech vaccine in patients with SARDs. These results highlight the need for additional mitigation strategies in this vulnerable population.
 Figure. Cumulative incidence curves (per 1,000 person-days) demonstrating time to development of breakthrough infection after vaccination.
Figure. Cumulative incidence curves (per 1,000 person-days) demonstrating time to development of breakthrough infection after vaccination..jpg) Table 1. Baseline clinical characteristics at the time of completion of initial SARS-CoV-2 vaccine series of patients with systemic autoimmune rheumatic diseases.
Table 1. Baseline clinical characteristics at the time of completion of initial SARS-CoV-2 vaccine series of patients with systemic autoimmune rheumatic diseases..jpg) Table 2. Clinical characteristics at time of vaccination associated with breakthrough infection among patients with systemic autoimmune rheumatic diseases.
Table 2. Clinical characteristics at time of vaccination associated with breakthrough infection among patients with systemic autoimmune rheumatic diseases.Disclosures: N. Patel, FVC Health; X. Wang, None; X. Fu, None; Y. Kawano, None; C. Cook, None; K. Vanni, None; G. Qian, None; E. Banasiak, None; E. Kowalski, None; y. zhang, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon.

