Back
Poster Session B
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1046–1070) Systemic Sclerosis and Related Disorders – Clinical Poster I
1049: Risk Stratification of Patients with Systemic Sclerosis-associated Pulmonary Arterial Hypertension in EUSTAR Using the Current and New Proposed Criteria
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Hilde Bjørkekjær, MD
Department of Rheumatology, Sørlandet sykehus HF, Kristiansand
Kristiansand, Norway
Abstract Poster Presenter(s)
Hilde Jenssen Bjørkekjær1, cosimo bruni2, PATRICIA E CARREIRA3, Paolo Airò4, CARMEN PILAR SIMEON5, Marie-Elise Truchetet6, Alessandro Giollo7, Alexandra Balbir-Gurman8, Mickaël MARTIN9, Chris Denton10, Armando Gabrielli11, Håvard Fretheim12, Imon Barua12, Helle Bitter13, Øyvind Midtvedt12, Kaspar Broch14, Arne Andreassen12, Yoshiya Tanaka15, Gabriela Riemekasten16, Ulf Müller-Ladner17, Marco Matucci-Cerinic2, Ivan Castellvi18, Elise Siegert19, Eric Hachulla20, Oliver Distler21 and Anna-Maria Hoffmann-Vold12, 1Department of Rheumatology, Hospital of Southern Norway, Kristiansand, Norway, Kristiansand, Norway, 2University of Florence, Florence, Italy, 3HOSPITAL 12 DE OCTUBRE, Madrid, Spain, 4Rheumatology and Clinical Immunology Unit, ASST Spedali Civili, Brescia, Italy, 5Hospital Vall D'Hebron, Barcelona, Spain, 6CHU de Bordeaux, Bordeaux, France, 7Rheumatology Section, Department of Medicine, University of Verona, Italy, Verona, Italy, 8Rheumatology Institute, Rambam Health Care Campus, Haifa, Israel, 9Poitiers's Universatory Hospital, Department of Internal Medicine, Poitiers, France, Mignaloux-Beauvoir, France, 10University College London, London, United Kingdom, 11Università Politecnica delle Marche, Ancona, Italy, 12Oslo University Hospital, Oslo, Norway, 13Sorlandet sykehus, Kristiansand, Norway, 14Oslo University Hospital, Rikshospitalet, Department of Cardiology, Oslo, Norway, Oslo, Norway, 15University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 16University Clinic Schleswit-Holstein (UKSH), Luebeck, Germany, 17JLU Campus KK, Bad Nauheim, Germany, 18Hospital Universitari de la Santa Creu i Sant Pau, Vilafranca del Pened, Spain, 19Charité Hospital, Berlin, Germany, 20University of Lille, LILLE, France, 21Department of Rheumatology, University Hospital Zurich, University of Zurich, Zürich, Switzerland
Background/Purpose: Pulmonary arterial hypertension (PAH) is a major clinical challenge in systemic sclerosis (SSc). A new definition for precapillary PH is proposed. Risk stratification is important for disease management, but is not validated in SSc-PAH and is not applied using the proposed criteria. We aimed to assess the performance of current risk stratification tools to estimate the 1- and 3- year mortality and to identify the best risk assessment approach in SSc-PAH.
Methods: We included all patients with newly diagnosed SSc-PAH according to the current and the proposed criteria from the European Scleroderma Trial and Research (EUSTAR) database from 2001 to 2021. The current criteria define PAH as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg, while the proposed criteria use mPAP >20 mmHg; a pulmonary artery wedge pressure ≤15 mmHg, and a pulmonary vascular resistance ≥3 Wood units in the absence of significant interstitial lung disease. We applied four different approaches for risk stratification at time of diagnosis including parameters according to the 2015 ESC/ERS Guidelines (Table).
1: Patients with ≥ 1 high-risk parameter were considered at high risk; ≥ 1 intermediate-risk parameter at intermediate risk, otherwise at low risk
2: Each parameter was graded from 1 to 3 representing low to high risk. The mean of available risk parameters was rounded to the nearest integer to define the risk category
3: Equals Model 2, but the intermediate risk group was divided into intermediate-low and intermediate-high based on the mean score
4: Stratifies patients into four risk categories based on the proportion of low-risk parameters
We used descriptive statistics and performed analysis of 1- and 3- year mortality in patients with a minimum follow-up of 1 and 3 years, respectively.
Results: Of 902 patients who conducted RHC, 271 (30%) were diagnosed with SSc-PAH according to the current criteria and an additional 36 (4%) according to the proposed criteria (Table).
Patients diagnosed according to the proposed criteria were diagnosed at a lower risk level compared with patients with PAH according to the current criteria (Figure 1).
For the current PAH criteria, the 1- and 3-year mortality was 10% and 31%. The models varied in their ability to estimate mortality (Figure 2). Models 1 and 4 either over- or underestimated mortality. Model 2 stratified patients according to the expected 1-year mortality of < 5%, 5-10% and >10% as suggested by the Guidelines. Model 3 segregated the mortality further within the intermediate-risk group.
For the patients who fulfilled the proposed PAH criteria, but not the current criteria, the 1- and 3-year mortality was 0% and 18%. The risk stratification models estimated mortality poorly, possibly due to the small sample size.
Conclusion: Models 2 and 3 seem to be well suited and provide signals for a better risk stratification of patients with newly diagnosed SSc-PAH according to the current criteria, with Model 3 further segregating the mortality in the intermediate-risk group. The patients who fulfilled the proposed PAH criteria, but not the established criteria, were on average diagnosed at a lower risk level, but the 3-year mortality is still high. This may provide guidance for optimized management in clinical practice.
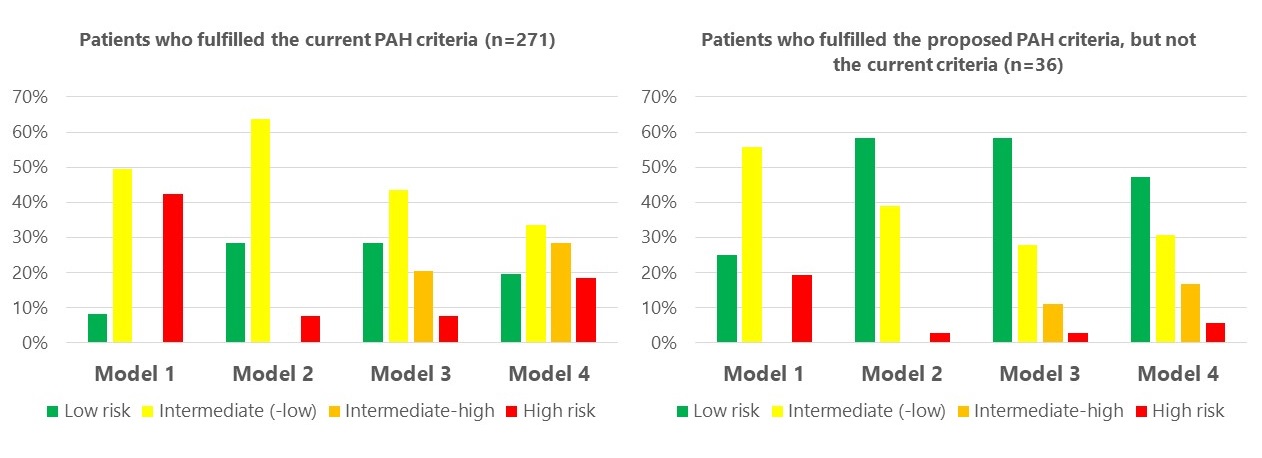 Figure 1: Proportion of patients in each risk category at baseline segregated by the different models
Figure 1: Proportion of patients in each risk category at baseline segregated by the different models
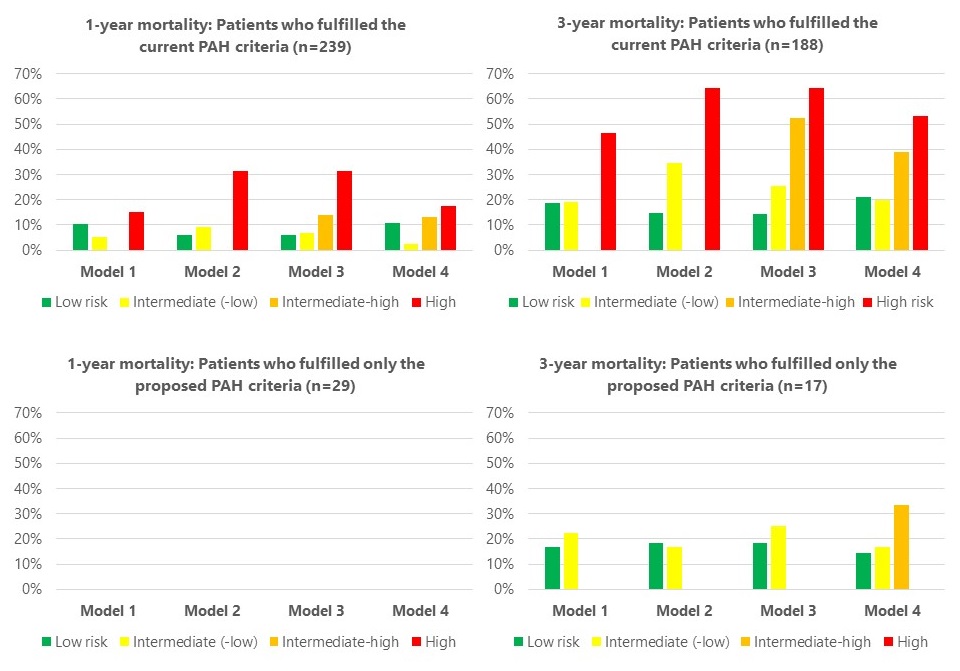 Figure 2: 1- and 3-year mortality according to risk category in the four different models in patients with a minimum follow-up of 1 and 3 years, respectively
Figure 2: 1- and 3-year mortality according to risk category in the four different models in patients with a minimum follow-up of 1 and 3 years, respectively
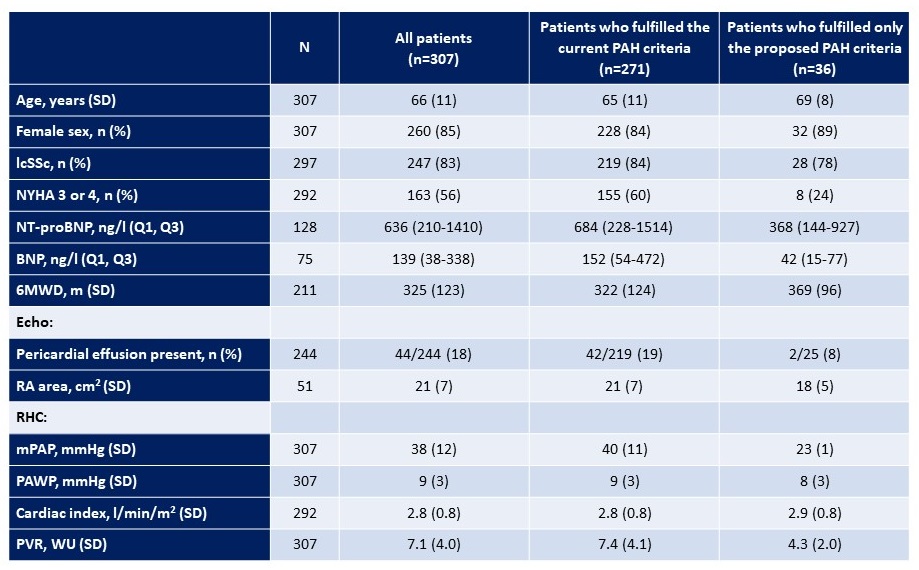 Table: Demographic and clinical characteristics at baseline of patients with PAH diagnosed according to the current and the proposed criteria.
Table: Demographic and clinical characteristics at baseline of patients with PAH diagnosed according to the current and the proposed criteria.
lcSSc: limited cutaneous systemic sclerosis; NYHA: New York Heart Association functional class; 6MWD: 6 minute walk distance; RA area: Right atrial area; RHC: Right heart catheterization; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance
Disclosures: H. Bjørkekjær, None; C. Bruni, Boehringer-Ingelheim, Eli Lilly; P. CARREIRA, None; P. Airò, Bristol-Myers-Squibb, Boehringer Ingelheim, Roche, Jannsen, CSL Behring; C. SIMEON, Merck/MSD, Janssen, Boehringer-Ingelheim; M. Truchetet, AbbVie/Abbott, Galapagos, Eli Lilly, Medac, Novartis, Pfizer, Roche; A. Giollo, None; A. Balbir-Gurman, None; M. MARTIN, None; C. Denton, Boehringer-Ingelheim, Roche, GlaxoSmithKlein(GSK), Horizon; A. Gabrielli, None; H. Fretheim, Bayer, GSK and Actelion; I. Barua, None; H. Bitter, Boehringer-Ingelheim; Ø. Midtvedt, Jannsen, Boehringer-Ingelheim; K. Broch, AstraZeneca, Bayer, Pfizer, Pharmacosmos, Vifor Pharma; A. Andreassen, Jannsen; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; G. Riemekasten, Boehringer Ingelheim; U. Müller-Ladner, Biogen; M. Matucci-Cerinic, Biogen, Eli Lilly, Chemomab, Sandoz, Merck/MSD, Pfizer, Behring, Janssen; I. Castellvi, None; E. Siegert, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; A. Hoffmann-Vold, Boehringer-Ingelheim, Janssen, Eli Lilly, Merck/MSD, Roche.
Background/Purpose: Pulmonary arterial hypertension (PAH) is a major clinical challenge in systemic sclerosis (SSc). A new definition for precapillary PH is proposed. Risk stratification is important for disease management, but is not validated in SSc-PAH and is not applied using the proposed criteria. We aimed to assess the performance of current risk stratification tools to estimate the 1- and 3- year mortality and to identify the best risk assessment approach in SSc-PAH.
Methods: We included all patients with newly diagnosed SSc-PAH according to the current and the proposed criteria from the European Scleroderma Trial and Research (EUSTAR) database from 2001 to 2021. The current criteria define PAH as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg, while the proposed criteria use mPAP >20 mmHg; a pulmonary artery wedge pressure ≤15 mmHg, and a pulmonary vascular resistance ≥3 Wood units in the absence of significant interstitial lung disease. We applied four different approaches for risk stratification at time of diagnosis including parameters according to the 2015 ESC/ERS Guidelines (Table).
1: Patients with ≥ 1 high-risk parameter were considered at high risk; ≥ 1 intermediate-risk parameter at intermediate risk, otherwise at low risk
2: Each parameter was graded from 1 to 3 representing low to high risk. The mean of available risk parameters was rounded to the nearest integer to define the risk category
3: Equals Model 2, but the intermediate risk group was divided into intermediate-low and intermediate-high based on the mean score
4: Stratifies patients into four risk categories based on the proportion of low-risk parameters
We used descriptive statistics and performed analysis of 1- and 3- year mortality in patients with a minimum follow-up of 1 and 3 years, respectively.
Results: Of 902 patients who conducted RHC, 271 (30%) were diagnosed with SSc-PAH according to the current criteria and an additional 36 (4%) according to the proposed criteria (Table).
Patients diagnosed according to the proposed criteria were diagnosed at a lower risk level compared with patients with PAH according to the current criteria (Figure 1).
For the current PAH criteria, the 1- and 3-year mortality was 10% and 31%. The models varied in their ability to estimate mortality (Figure 2). Models 1 and 4 either over- or underestimated mortality. Model 2 stratified patients according to the expected 1-year mortality of < 5%, 5-10% and >10% as suggested by the Guidelines. Model 3 segregated the mortality further within the intermediate-risk group.
For the patients who fulfilled the proposed PAH criteria, but not the current criteria, the 1- and 3-year mortality was 0% and 18%. The risk stratification models estimated mortality poorly, possibly due to the small sample size.
Conclusion: Models 2 and 3 seem to be well suited and provide signals for a better risk stratification of patients with newly diagnosed SSc-PAH according to the current criteria, with Model 3 further segregating the mortality in the intermediate-risk group. The patients who fulfilled the proposed PAH criteria, but not the established criteria, were on average diagnosed at a lower risk level, but the 3-year mortality is still high. This may provide guidance for optimized management in clinical practice.
 Figure 1: Proportion of patients in each risk category at baseline segregated by the different models
Figure 1: Proportion of patients in each risk category at baseline segregated by the different models Figure 2: 1- and 3-year mortality according to risk category in the four different models in patients with a minimum follow-up of 1 and 3 years, respectively
Figure 2: 1- and 3-year mortality according to risk category in the four different models in patients with a minimum follow-up of 1 and 3 years, respectively Table: Demographic and clinical characteristics at baseline of patients with PAH diagnosed according to the current and the proposed criteria.
Table: Demographic and clinical characteristics at baseline of patients with PAH diagnosed according to the current and the proposed criteria.lcSSc: limited cutaneous systemic sclerosis; NYHA: New York Heart Association functional class; 6MWD: 6 minute walk distance; RA area: Right atrial area; RHC: Right heart catheterization; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance
Disclosures: H. Bjørkekjær, None; C. Bruni, Boehringer-Ingelheim, Eli Lilly; P. CARREIRA, None; P. Airò, Bristol-Myers-Squibb, Boehringer Ingelheim, Roche, Jannsen, CSL Behring; C. SIMEON, Merck/MSD, Janssen, Boehringer-Ingelheim; M. Truchetet, AbbVie/Abbott, Galapagos, Eli Lilly, Medac, Novartis, Pfizer, Roche; A. Giollo, None; A. Balbir-Gurman, None; M. MARTIN, None; C. Denton, Boehringer-Ingelheim, Roche, GlaxoSmithKlein(GSK), Horizon; A. Gabrielli, None; H. Fretheim, Bayer, GSK and Actelion; I. Barua, None; H. Bitter, Boehringer-Ingelheim; Ø. Midtvedt, Jannsen, Boehringer-Ingelheim; K. Broch, AstraZeneca, Bayer, Pfizer, Pharmacosmos, Vifor Pharma; A. Andreassen, Jannsen; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; G. Riemekasten, Boehringer Ingelheim; U. Müller-Ladner, Biogen; M. Matucci-Cerinic, Biogen, Eli Lilly, Chemomab, Sandoz, Merck/MSD, Pfizer, Behring, Janssen; I. Castellvi, None; E. Siegert, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; O. Distler, AbbVie/Abbott, Amgen, GlaxoSmithKlein(GSK), Novartis, Roche, UCB, Kymera, Mitsubishi Tanabe, Boehringer Ingelheim, 4P-Pharma, Acceleron, Alcimed, Altavant Sciences, AnaMar, Arxx, AstraZeneca, Blade Therapeutics, Bayer, Corbus Pharmaceuticals, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Lupin, Miltenyi Biotec, Merck/MSD, Prometheus Biosciences, Redx Pharma, Roivant, Sanofi, Topadur, Pfizer, Janssen, Medscape, Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143), FOREUM Foundation, ERS/EULAR Guidelines, EUSTAR, SCQM (Swiss Clinical Quality Management in Rheumatic Diseases), Swiss Academy of Medical Sciences (SAMW), Hartmann Müller Foundation; A. Hoffmann-Vold, Boehringer-Ingelheim, Janssen, Eli Lilly, Merck/MSD, Roche.

