Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0695–0723) Epidemiology and Public Health Poster I
0715: Safety and Immunogenicity of Mixed COVID19 Vaccine Regimens in Immune-mediated Inflammatory Diseases: An Observational Cohort
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CH
Carol Hitchon, MD, MSc
University of Manitoba
Winnipeg, MB, Canada
Abstract Poster Presenter(s)
Carol Hitchon1, Christine Mesa2, Charles N Bernstein1, Catherine Card2, RuthAnn Marrie1, Sheila O'Brien3 and John Kim2, 1University of Manitoba, Winnipeg, MB, Canada, 2Public Health Agency of Canada, Winnipeg, MB, Canada, 3Canada Blood Services, Ottawa, Canada
Background/Purpose: Data on immunogenicity and safety of COVID-19 vaccination strategies exist for the general population, however, such data are limited for immunocompromised people including those with immune-mediated inflammatory diseases (IMIDs). Among persons with IMIDs who received homologous or heterologous SARS-CoV-2 vaccines, we compared post-vaccine IMID disease activity and vaccine antibody responses including responses after a third vaccine (V3).
Methods: From 02/2021-03/2022 persons diagnosed with any of inflammatory arthritis (n= 66; 77% rheumatoid arthritis), systemic autoimmune rheumatic diseases (n=82; 63% lupus), inflammatory bowel disease (n= 89; 43% Crohn's), and multiple sclerosis (n= 72; 77% relapsing remitting) self-reported COVID-19 illness and exposure risks, IMID disease activity/state using disease-specific measures, and had anti-spike, -receptor binding domain (RBD) and -nucleocapsid (NC) IgG antibodies tested by multiplex immunoassays following each vaccination (V1, V2, V3). Anti-SARS-CoV-2 responses were compared across vaccine regimens and to responses in 370 age-sex matched vaccinated blood donor controls. Variables associated with seroconversion 1 month post-V2 were tested using binary logistic regression models that included age, sex, diagnosis, vaccine interval (< or > 28 days), and IMID treatment.
Results: IMID participants were predominantly female (79%), white (95%), with a mean (standard deviation) age of 56.3 (14.2) years and a median (range) of 2 (0-9) comorbidities; 23% were taking immunosuppressants, 28% biologics, and 27% other immunomodulators. Only 1.6% had confirmed COVID-19 by community-based polymerase chain reaction testing prior to Jan 2022. For their initial vaccination course (V1 and V2), most participants (66.2%) received homologous mRNA (BNT162b2 or mRNA1273) vaccines, 1.9% received homologous ChAdOx1, and 31.9% received heterologous vaccines (24.2% ChAdOx1/mRNA, 5.6% heterologous mRNA). IMID disease activity/state was similar before and after V1 and V2. Seroconversion rates for 238 IMIDs increased 1 month post V2 (post V1 anti-spike 52%, anti-RBD 59%; post V2 anti-spike 90%; anti-RBD 92%), remained lower than controls (V2 anti-Spike 98.1% p< 0.0001). Antibody titers waned by 3 months but increased post-V3 (Figure 1). If primed with a vector vaccine, providing a mRNA vaccine as the second vaccine increased antibody titers to those comparable to homologous mRNA vaccines. Participants over age 65 years, diagnosed with MS, taking biologics, or having early vaccination (< 28 days between V1 and V2) were less likely to seroconvert in multivariate models Table. Most IMIDs who did not seroconvert were taking immunosuppressives (mycophenolate n=8; methotrexate n= 2, azathioprine n=2) or biologics (B cell targeting current/past n=12, anti-TNF n=4, other n= 1).
Conclusion: Heterologous COVID-19 vaccination improves seroconversion rates following a viral vector vaccine and does not lead to disease flare in IMID patients. Persons with IMIDs benefit from booster vaccination.
.jpg) Figure 1: Anti-SARS-CoV2 IgG levels following vaccination.
Figure 1: Anti-SARS-CoV2 IgG levels following vaccination.
A. log anti-Spike B. log anti-RBD
Data for seroconverters only. RBD= receptor binding domain; IA=inflammatory arthritis; SARDS= systemic autoimmune rheumatic disease; IBD= inflammatory bowel disease; MS= multiple sclerosis. * p < 0.0001
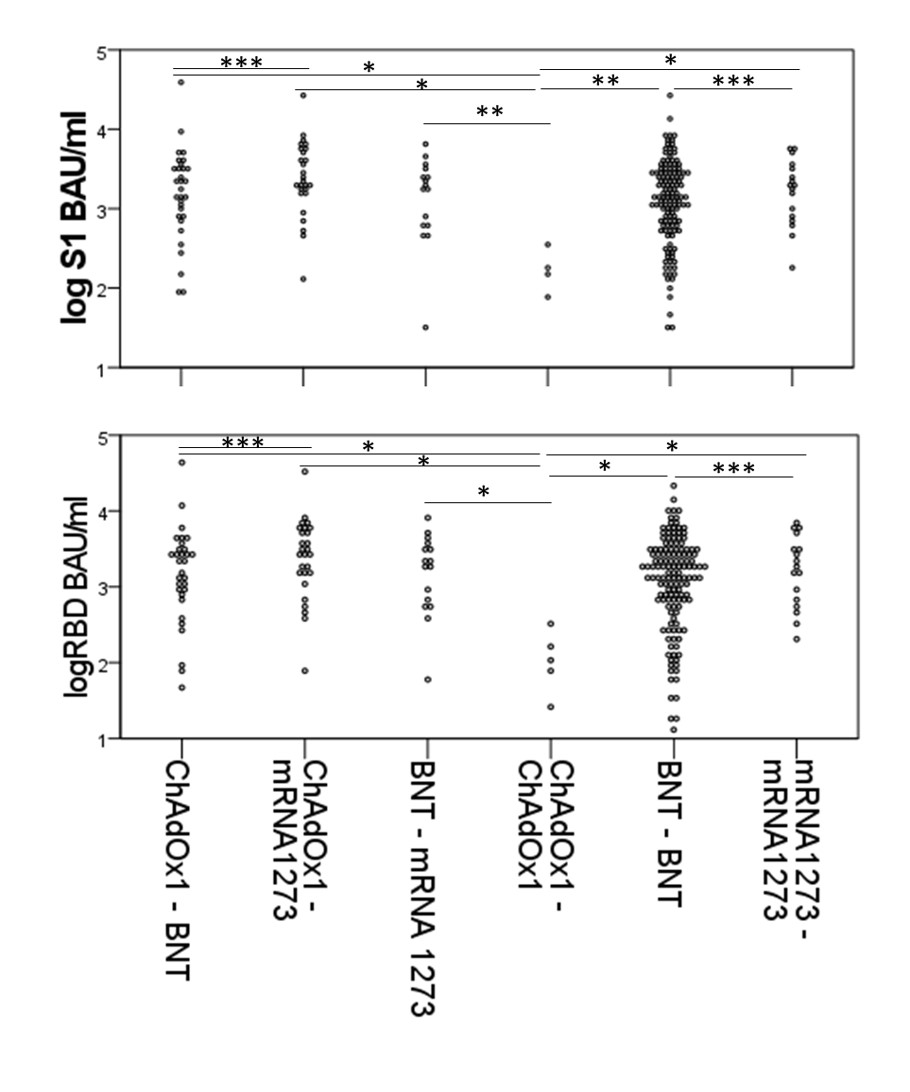 Figure 2 Antibody levels 1 month following second vaccination by vaccine mixture.
Figure 2 Antibody levels 1 month following second vaccination by vaccine mixture.
A log anti-RBD B log anti-Spike
Data for seroconverters only. BNT= BNT162b2, Bonferroni Adjusted p values * p < 0.005, ** p < 0.05, *** p=NS
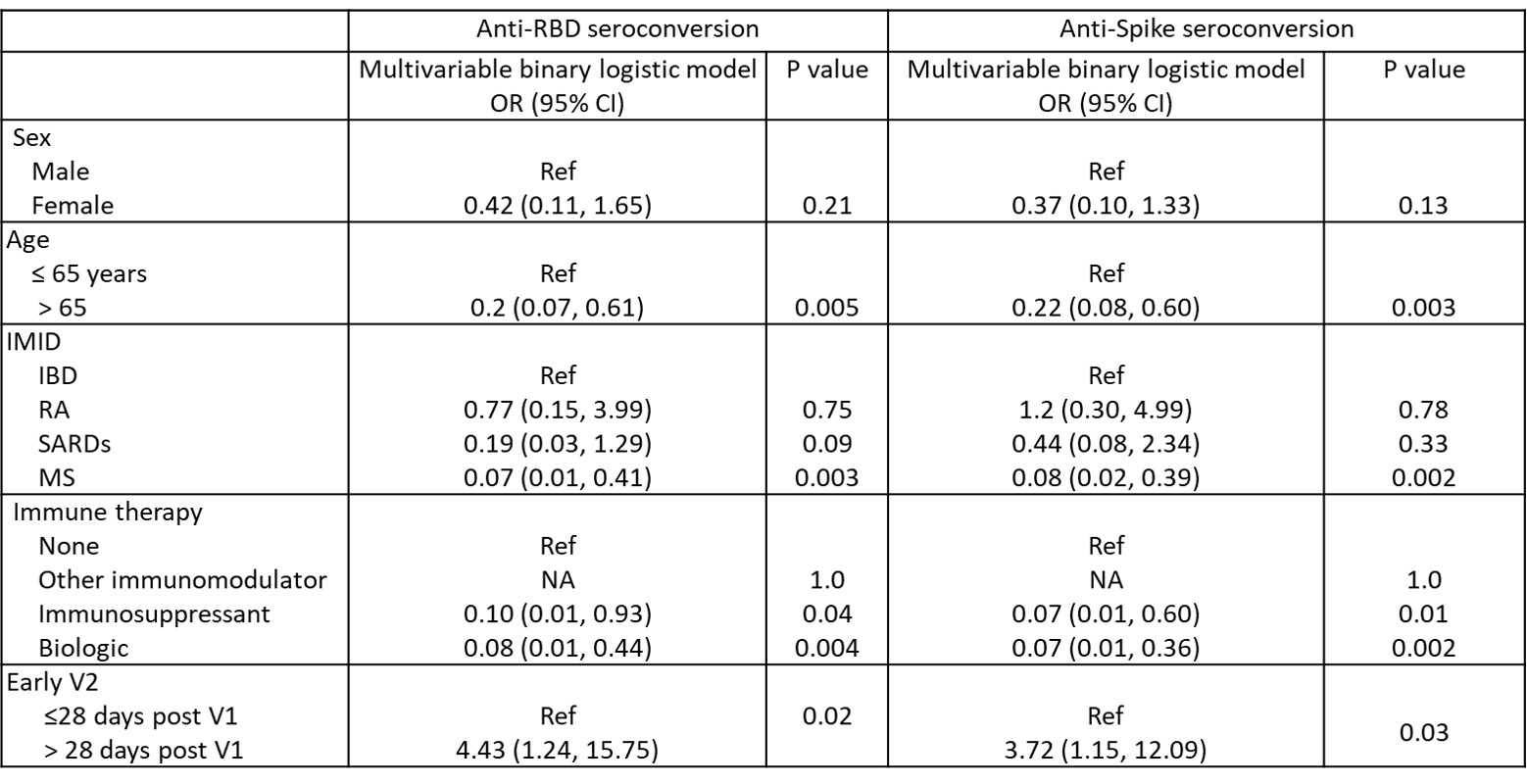 Table:Clinical variables associated with seroconversion post V2.
Table:Clinical variables associated with seroconversion post V2.
RBD = Receptor binding domain; S1 = Spike protein; Ref = reference category; NA = not able to compute.
Disclosures: C. Hitchon, Pfizer Canada, Astra-Zeneca Canada, Pfizer; C. Mesa, None; C. Bernstein, AbbVie Canada, Amgen Canada, Bristol Myers Squibb Canada, Roche Canada, Janssen Canada, Sandoz Canada, Takeda Canada, Mylan Pharmaceuticals, Pfizer Canada; C. Card, None; R. Marrie, Biogen Idec, Roche Canada; S. O'Brien, None; J. Kim, None.
Background/Purpose: Data on immunogenicity and safety of COVID-19 vaccination strategies exist for the general population, however, such data are limited for immunocompromised people including those with immune-mediated inflammatory diseases (IMIDs). Among persons with IMIDs who received homologous or heterologous SARS-CoV-2 vaccines, we compared post-vaccine IMID disease activity and vaccine antibody responses including responses after a third vaccine (V3).
Methods: From 02/2021-03/2022 persons diagnosed with any of inflammatory arthritis (n= 66; 77% rheumatoid arthritis), systemic autoimmune rheumatic diseases (n=82; 63% lupus), inflammatory bowel disease (n= 89; 43% Crohn's), and multiple sclerosis (n= 72; 77% relapsing remitting) self-reported COVID-19 illness and exposure risks, IMID disease activity/state using disease-specific measures, and had anti-spike, -receptor binding domain (RBD) and -nucleocapsid (NC) IgG antibodies tested by multiplex immunoassays following each vaccination (V1, V2, V3). Anti-SARS-CoV-2 responses were compared across vaccine regimens and to responses in 370 age-sex matched vaccinated blood donor controls. Variables associated with seroconversion 1 month post-V2 were tested using binary logistic regression models that included age, sex, diagnosis, vaccine interval (< or > 28 days), and IMID treatment.
Results: IMID participants were predominantly female (79%), white (95%), with a mean (standard deviation) age of 56.3 (14.2) years and a median (range) of 2 (0-9) comorbidities; 23% were taking immunosuppressants, 28% biologics, and 27% other immunomodulators. Only 1.6% had confirmed COVID-19 by community-based polymerase chain reaction testing prior to Jan 2022. For their initial vaccination course (V1 and V2), most participants (66.2%) received homologous mRNA (BNT162b2 or mRNA1273) vaccines, 1.9% received homologous ChAdOx1, and 31.9% received heterologous vaccines (24.2% ChAdOx1/mRNA, 5.6% heterologous mRNA). IMID disease activity/state was similar before and after V1 and V2. Seroconversion rates for 238 IMIDs increased 1 month post V2 (post V1 anti-spike 52%, anti-RBD 59%; post V2 anti-spike 90%; anti-RBD 92%), remained lower than controls (V2 anti-Spike 98.1% p< 0.0001). Antibody titers waned by 3 months but increased post-V3 (Figure 1). If primed with a vector vaccine, providing a mRNA vaccine as the second vaccine increased antibody titers to those comparable to homologous mRNA vaccines. Participants over age 65 years, diagnosed with MS, taking biologics, or having early vaccination (< 28 days between V1 and V2) were less likely to seroconvert in multivariate models Table. Most IMIDs who did not seroconvert were taking immunosuppressives (mycophenolate n=8; methotrexate n= 2, azathioprine n=2) or biologics (B cell targeting current/past n=12, anti-TNF n=4, other n= 1).
Conclusion: Heterologous COVID-19 vaccination improves seroconversion rates following a viral vector vaccine and does not lead to disease flare in IMID patients. Persons with IMIDs benefit from booster vaccination.
.jpg) Figure 1: Anti-SARS-CoV2 IgG levels following vaccination.
Figure 1: Anti-SARS-CoV2 IgG levels following vaccination. A. log anti-Spike B. log anti-RBD
Data for seroconverters only. RBD= receptor binding domain; IA=inflammatory arthritis; SARDS= systemic autoimmune rheumatic disease; IBD= inflammatory bowel disease; MS= multiple sclerosis. * p < 0.0001
 Figure 2 Antibody levels 1 month following second vaccination by vaccine mixture.
Figure 2 Antibody levels 1 month following second vaccination by vaccine mixture. A log anti-RBD B log anti-Spike
Data for seroconverters only. BNT= BNT162b2, Bonferroni Adjusted p values * p < 0.005, ** p < 0.05, *** p=NS
 Table:Clinical variables associated with seroconversion post V2.
Table:Clinical variables associated with seroconversion post V2.RBD = Receptor binding domain; S1 = Spike protein; Ref = reference category; NA = not able to compute.
Disclosures: C. Hitchon, Pfizer Canada, Astra-Zeneca Canada, Pfizer; C. Mesa, None; C. Bernstein, AbbVie Canada, Amgen Canada, Bristol Myers Squibb Canada, Roche Canada, Janssen Canada, Sandoz Canada, Takeda Canada, Mylan Pharmaceuticals, Pfizer Canada; C. Card, None; R. Marrie, Biogen Idec, Roche Canada; S. O'Brien, None; J. Kim, None.

