Back
Poster Session B
Infection-related rheumatic syndromes
Session: (0779–0806) Infection-related Rheumatic Disease Poster
0796: SARS-CoV-2 Vaccine in Axial Spondyloarthritis and Psoriatic Arthritis: Does Sulfasalazine Counterbalance TNFI Impaired Immunogenicity?
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CS
Carla Goncalves Schahin Saad, MD, PhD
Faculdade de Medicina da Universidade de Sao Paulo
Sao Paulo, S�o Paulo, Brazil
Abstract Poster Presenter(s)
Carla Goncalves Schahin Saad1, Matheus SR Silva2, PERCIVAL SAMPAIO-BARROS1, Julio Moraes3, Claudia G Schainberg2, Celio Roberto goncalves1, Andrea Shimabuco1, Nadia Aikawa1, Emily Figueiredo Neves Yuki1, Sandra Gofinet Pasoto4, Leonard VK Kupa2, Renato K Aoyama2, Carlo Araujo1, Clovis A. Silva5, ana Medeiros6 and Eloisa Silva Dutra de Oliveira Bonfa4, 1Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil, 2Faculdade de Medicina da Universidade de São Paulo, São Paulo, 3Faculdade de Medicina da Universidade de Sao Paulo, Jundiai, Brazil, 4Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil, 5Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, Brazil, 6Faculdade de Medicina da Universidade de São Paulo, São Jose Dos Campos, Brazil
Background/Purpose: Spondyloarthritis(SpA) patients are exposed to a variety of immunosuppressors capable of reduce humoral responses to vaccination. In context of the SARS-CoV-2 pandemic, assessment of the factors related with better or worse humoral responses is paramount to a better management of these patients. We therefore aimed to evaluate humoral responses to three doses of the inactivated SARS-CoV-2 vaccine(CoronaVac) in patients with spondyloarthritis(SpA) and the effect of therapy, compared with a control group(CG).
Methods: Prospective cohort of axial SpA/psoriatic arthritis patients and age/sex-balanced CG from the (CoronavRheum, clinicaltrials.gov #NCT04754698). CoronaVac was given in two doses(28-days interval) with a booster at day 210. Blood samples were collected in the days 0/28(D28)/69(D69) and 240(D240) to evaluate anti-SARS-CoV-2 IgG seropositivity(SP) and neutralising antibodies(NAb).
Results: 194 SpA patients were enrolled and 183 patients were age/sex-balanced with 183 CG. At D69, SpA patients showed a high SP (80·2% vs. 95·7%, p< 0·001) and moderate NAb positivity (61·6% vs. 82·7%, p< 0·001), but lower than CG. In patients, older age (p=0·038), prednisone (p< 0·001), methotrexate (p< 0·001) and TNFi (p< 0·001) were independently associated with lower SP, while Caucasian race (p=0·047) and prednisone (p=0·008) were associated with diminished NAb. In contrast, sulfasalazine (SSZ) use was associated with NAb presence (p=0·042). In monotherapy, only TNFi was also associated with absence of SP (p=0·035). Further comparison with CG revealed that TNFi and/or MTX negatively impacted SP/NAb (p< 0·05). In contrast, patients under SSZ monotherapy achieved 100% SP (p >0·999) and 83·3% NAb positivity (p >0·999). SSZ+TNFi combination resulted in a similar response than CG [SP (p=0·153) and NAb (p=0·715)]. After third dose(D69-D240), a major increment occurred for SP (81·3% to 93·1%,p< 0·001) and NAb (63·2% to 86·1%,p< 0·001), but still lower than CG (p< 0·05), and only TNFi impaired both SP (p=0·016) and NAb (p=0·002).
Conclusion: We provided novel data demonstrating that TNFi reduces immunogenicity in SpA patients while SSZ has a positive impact on vaccine antibody response. Further studies are necessary to confirm whether the concomitant use of SSZ may counterbalance the negative effect of TNFi.
This study was sponsored by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (#2015/03756–4 to N.E.A., C.A.S., S.G.P. and E.B; #2019/17272-0 to L.V.K.K), Conselho Nacional de Desenvolvimento Científico e Tecnológico (#304984/2020-5 to C.A.S. and #305242/2019-9 to E.B.), B3-Bolsa de Valores do Brasil and Instituto Todos pela Saúde (ITPS 01/2021, C1313 to N.E.A., C.A.S. S.G.P and E.B.). Instituto Butantan supplied the study product and had no other role in the trial.
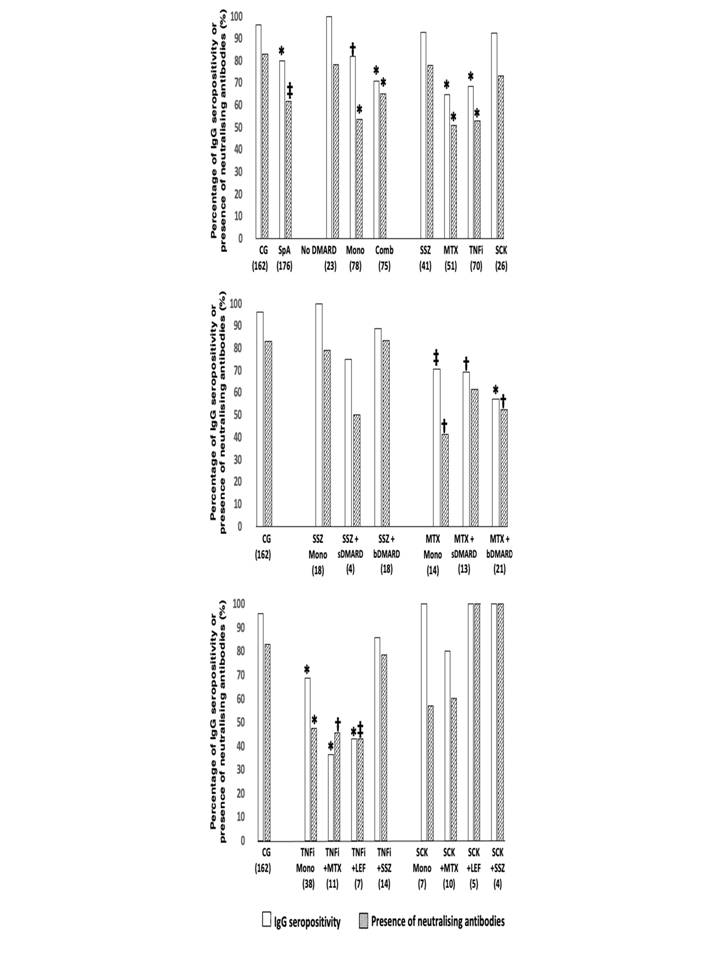 Anti-SARS-CoV-2 S1/S2 IgG seropositivity and presence of neutralising antibodies at D69, according to spondyloarthritis (SpA) treatments in comparison to control group (CG), using a two-sided chi-square or Fisher’s exact test, as appropriate. All the analyses are two-sided. Data are shown as percentages. CG - control group; SpA - spondyloarthritis; DMARD - disease modifying antirheumatic drugs; Mono - DMARD monotherapy and without corticosteroids; Comb - combination therapy of DMARD; SSZ – sulfasalazine; MTX - methotrexate; TNFi - tumor necrosis factor inhibitors; SCK – secukinumab; sDMARD – synthetic disease modifying antirheumatic drugs; bDMARD – biological disease modifying antirheumatic drugs; LEF – leflunomide; * p < 0.001 vs. CG; † p < 0.01 vs. CG; ‡ p < 0.05 vs. CG. The number of patients in groups is depicted under their designations.
Anti-SARS-CoV-2 S1/S2 IgG seropositivity and presence of neutralising antibodies at D69, according to spondyloarthritis (SpA) treatments in comparison to control group (CG), using a two-sided chi-square or Fisher’s exact test, as appropriate. All the analyses are two-sided. Data are shown as percentages. CG - control group; SpA - spondyloarthritis; DMARD - disease modifying antirheumatic drugs; Mono - DMARD monotherapy and without corticosteroids; Comb - combination therapy of DMARD; SSZ – sulfasalazine; MTX - methotrexate; TNFi - tumor necrosis factor inhibitors; SCK – secukinumab; sDMARD – synthetic disease modifying antirheumatic drugs; bDMARD – biological disease modifying antirheumatic drugs; LEF – leflunomide; * p < 0.001 vs. CG; † p < 0.01 vs. CG; ‡ p < 0.05 vs. CG. The number of patients in groups is depicted under their designations.
Disclosures: C. Saad, None; M. Silva, None; P. SAMPAIO-BARROS, None; J. Moraes, None; C. Schainberg, None; C. goncalves, None; A. Shimabuco, None; N. Aikawa, None; E. Figueiredo Neves Yuki, None; S. Gofinet Pasoto, None; L. Kupa, None; R. Aoyama, None; C. Araujo, None; C. Silva, None; a. Medeiros, None; E. Silva Dutra de Oliveira Bonfa, None.
Background/Purpose: Spondyloarthritis(SpA) patients are exposed to a variety of immunosuppressors capable of reduce humoral responses to vaccination. In context of the SARS-CoV-2 pandemic, assessment of the factors related with better or worse humoral responses is paramount to a better management of these patients. We therefore aimed to evaluate humoral responses to three doses of the inactivated SARS-CoV-2 vaccine(CoronaVac) in patients with spondyloarthritis(SpA) and the effect of therapy, compared with a control group(CG).
Methods: Prospective cohort of axial SpA/psoriatic arthritis patients and age/sex-balanced CG from the (CoronavRheum, clinicaltrials.gov #NCT04754698). CoronaVac was given in two doses(28-days interval) with a booster at day 210. Blood samples were collected in the days 0/28(D28)/69(D69) and 240(D240) to evaluate anti-SARS-CoV-2 IgG seropositivity(SP) and neutralising antibodies(NAb).
Results: 194 SpA patients were enrolled and 183 patients were age/sex-balanced with 183 CG. At D69, SpA patients showed a high SP (80·2% vs. 95·7%, p< 0·001) and moderate NAb positivity (61·6% vs. 82·7%, p< 0·001), but lower than CG. In patients, older age (p=0·038), prednisone (p< 0·001), methotrexate (p< 0·001) and TNFi (p< 0·001) were independently associated with lower SP, while Caucasian race (p=0·047) and prednisone (p=0·008) were associated with diminished NAb. In contrast, sulfasalazine (SSZ) use was associated with NAb presence (p=0·042). In monotherapy, only TNFi was also associated with absence of SP (p=0·035). Further comparison with CG revealed that TNFi and/or MTX negatively impacted SP/NAb (p< 0·05). In contrast, patients under SSZ monotherapy achieved 100% SP (p >0·999) and 83·3% NAb positivity (p >0·999). SSZ+TNFi combination resulted in a similar response than CG [SP (p=0·153) and NAb (p=0·715)]. After third dose(D69-D240), a major increment occurred for SP (81·3% to 93·1%,p< 0·001) and NAb (63·2% to 86·1%,p< 0·001), but still lower than CG (p< 0·05), and only TNFi impaired both SP (p=0·016) and NAb (p=0·002).
Conclusion: We provided novel data demonstrating that TNFi reduces immunogenicity in SpA patients while SSZ has a positive impact on vaccine antibody response. Further studies are necessary to confirm whether the concomitant use of SSZ may counterbalance the negative effect of TNFi.
This study was sponsored by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (#2015/03756–4 to N.E.A., C.A.S., S.G.P. and E.B; #2019/17272-0 to L.V.K.K), Conselho Nacional de Desenvolvimento Científico e Tecnológico (#304984/2020-5 to C.A.S. and #305242/2019-9 to E.B.), B3-Bolsa de Valores do Brasil and Instituto Todos pela Saúde (ITPS 01/2021, C1313 to N.E.A., C.A.S. S.G.P and E.B.). Instituto Butantan supplied the study product and had no other role in the trial.
 Anti-SARS-CoV-2 S1/S2 IgG seropositivity and presence of neutralising antibodies at D69, according to spondyloarthritis (SpA) treatments in comparison to control group (CG), using a two-sided chi-square or Fisher’s exact test, as appropriate. All the analyses are two-sided. Data are shown as percentages. CG - control group; SpA - spondyloarthritis; DMARD - disease modifying antirheumatic drugs; Mono - DMARD monotherapy and without corticosteroids; Comb - combination therapy of DMARD; SSZ – sulfasalazine; MTX - methotrexate; TNFi - tumor necrosis factor inhibitors; SCK – secukinumab; sDMARD – synthetic disease modifying antirheumatic drugs; bDMARD – biological disease modifying antirheumatic drugs; LEF – leflunomide; * p < 0.001 vs. CG; † p < 0.01 vs. CG; ‡ p < 0.05 vs. CG. The number of patients in groups is depicted under their designations.
Anti-SARS-CoV-2 S1/S2 IgG seropositivity and presence of neutralising antibodies at D69, according to spondyloarthritis (SpA) treatments in comparison to control group (CG), using a two-sided chi-square or Fisher’s exact test, as appropriate. All the analyses are two-sided. Data are shown as percentages. CG - control group; SpA - spondyloarthritis; DMARD - disease modifying antirheumatic drugs; Mono - DMARD monotherapy and without corticosteroids; Comb - combination therapy of DMARD; SSZ – sulfasalazine; MTX - methotrexate; TNFi - tumor necrosis factor inhibitors; SCK – secukinumab; sDMARD – synthetic disease modifying antirheumatic drugs; bDMARD – biological disease modifying antirheumatic drugs; LEF – leflunomide; * p < 0.001 vs. CG; † p < 0.01 vs. CG; ‡ p < 0.05 vs. CG. The number of patients in groups is depicted under their designations. Disclosures: C. Saad, None; M. Silva, None; P. SAMPAIO-BARROS, None; J. Moraes, None; C. Schainberg, None; C. goncalves, None; A. Shimabuco, None; N. Aikawa, None; E. Figueiredo Neves Yuki, None; S. Gofinet Pasoto, None; L. Kupa, None; R. Aoyama, None; C. Araujo, None; C. Silva, None; a. Medeiros, None; E. Silva Dutra de Oliveira Bonfa, None.

