Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0636: Serum Soluble Mediator Signatures of Lupus Nephritis Histological Features and Response to Treatment
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Andrea Fava, MD
Johns Hopkins University
Baltimore, MD, United States
Abstract Poster Presenter(s)
Andrea Fava1, Carla J. Guthridge2, Joseph Kheir2, Catriona Wagner3, Michelle Petri4, Jill Buyon5, Betty Diamond6, the Accelerating Medicines Partnership (AMP) RA/SLE7, Joel Guthridge2 and Judith James2, 1Johns Hopkins University, Baltimore, MD, 2Oklahoma Medical Research Foundation, Oklahoma City, OK, 3Oklahoma Medical Research Foundation, Oklahoma, 4Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 5NYU Grossman School of Medicine, New York, NY, 6Feinstein Institutes for Medical Research, Manhasset, NY, 7Multiple Insitutions
Background/Purpose: There is a pressing need to identify novel therapeutic approaches and noninvasive biomarkers in lupus nephritis (LN). In this study, we quantified serum soluble mediators in the large Accelerating Medicines Partnership (AMP) LN longitudinal cohort to identify novel biomarkers of histological features and treatment response and provide insights into the pathogenesis of LN.
Methods: SLE patients meeting ACR or SLICC criteria (n=268) undergoing a clinically indicated kidney biopsy with a urine protein/creatinine (UPCR) ≥0.5 were recruited for this study as part of the AMP. Serum samples were collected from patients at the time of diagnostic kidney biopsy and 3-, 6-, and 12-months post-biopsy and from 22 healthy donors (HD). Concentrations of 51 analytes, including cytokines, chemokines, and TNFR superfamily members, were analyzed by xMAP multiplex assays on the Bio-Rad BioPlex200® array system. TACE levels were determined by ELISA. Clinical response was determined at 12-months post-biopsy using the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study definitions in patients with a baseline UPCR >1.0 and International Society of Nephrology/Renal Pathology Society class III, IV, V, or combination thereof.
Results: LN patients demonstrated heterogeneity in serum soluble mediator concentrations (Fig 1). Most soluble mediators were elevated in LN patients compared to HD (Fig. 2A). Within LN, patients with proliferative LN (class III +/- V or class IV +/-V) displayed a distinct signature (Fig 2B). In particular, patients with pure proliferative LN (class III or IV) had higher serum levels of immune mediators, such as IFNβ and IL-1β, compared to nonproliferative LN (Fig 2C), including pure membranous (class V) LN (Fig 2D). In addition, several serum immune mediators correlated with intrarenal LN activity (NIH activity index), with syndecan-1 (CD138) and TNF-RII exhibiting the highest correlation (Fig 3A). In contrast, stem cell factor (SCF) and TNF-RI had the highest correlation with the chronicity index (Fig 2B). In proliferative LN, the concentrations of many immune mediators declined in treatment responders (not shown). For example, syndecan-1 and TNF-RII decreased in complete and partial responders but not in nonresponders (Fig 3C-D). In contrast, SCF levels remained relatively stable regardless of response status (Fig 3E).
Conclusion: Distinct histological features of LN are paralleled by specific signatures of circulating soluble mediators. Within LN, proliferative LN is associated with higher circulating levels of inflammatory cytokines, notably, type 1 IFNs. Furthermore, a decline in the titers of several immune mediators correlated with LN activity was associated with treatment response, suggesting a possible role in LN pathogenesis. These signatures and trajectories provide insight into LN pathogenesis, heterogeneity, and biomarker development.
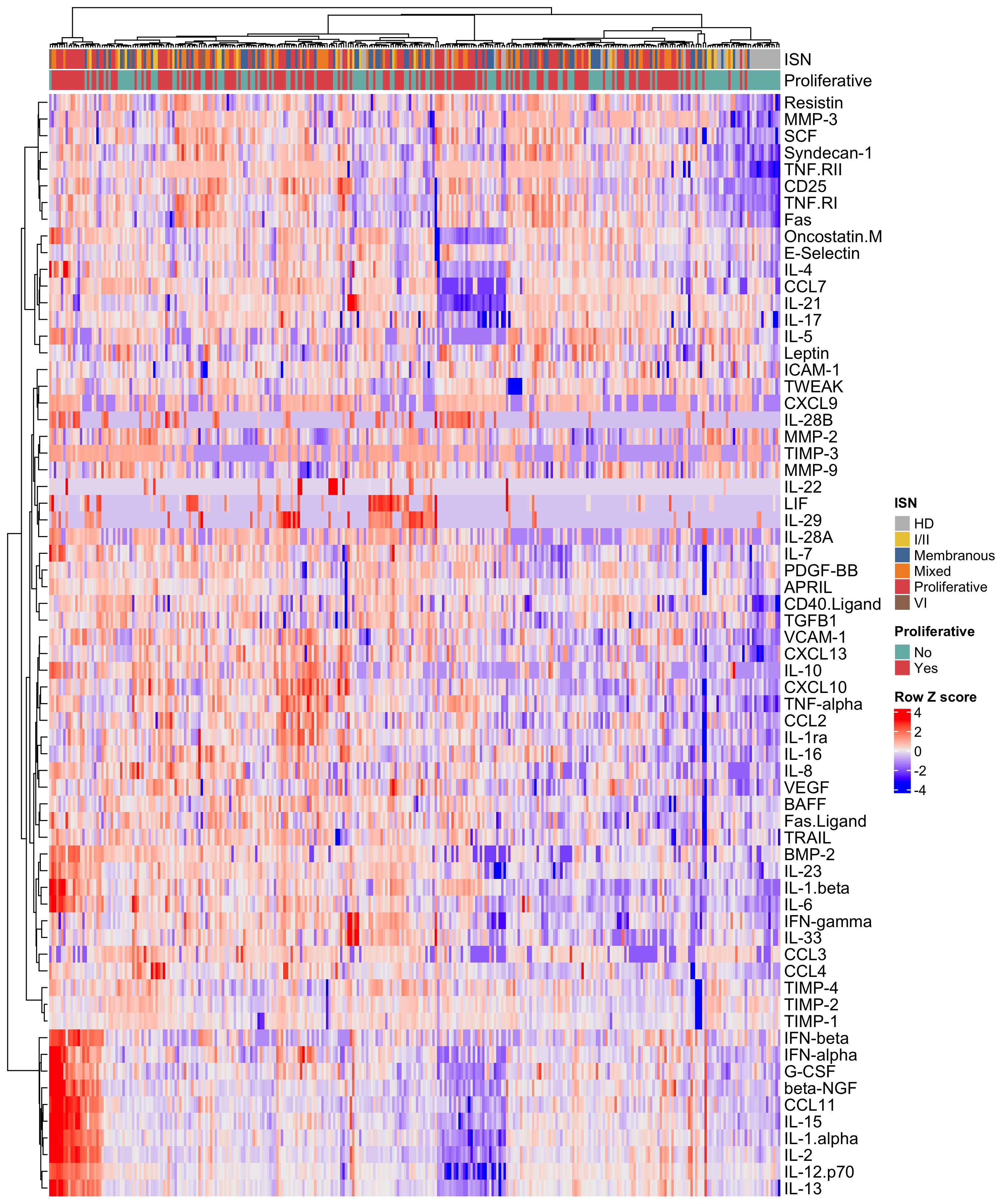 Figure 1. Soluble mediator heterogeneity in lupus nephritis (LN) patients. Heatmap of the soluble mediator concentrations in healthy donors (HD) and LN patients. International Society of Nephrology/Renal Pathology Society (ISN) class and proliferative versus non-proliferative LN are indicated by bars on the top of the heatmap.
Figure 1. Soluble mediator heterogeneity in lupus nephritis (LN) patients. Heatmap of the soluble mediator concentrations in healthy donors (HD) and LN patients. International Society of Nephrology/Renal Pathology Society (ISN) class and proliferative versus non-proliferative LN are indicated by bars on the top of the heatmap.
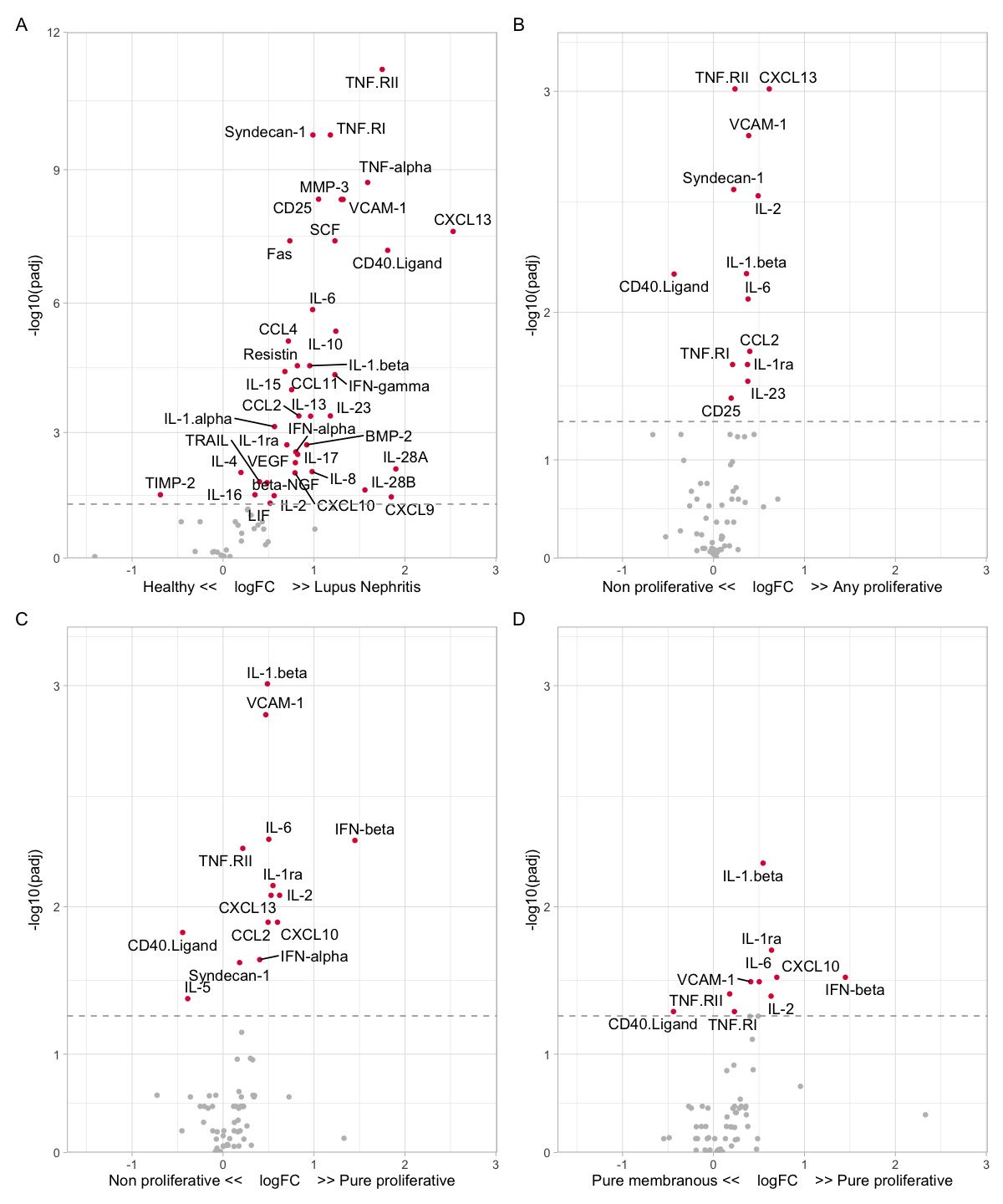 Figure 2. Soluble mediator profiles of lupus nephritis (LN) and proliferative LN. Volcano plots demonstrating the differential abundance of soluble mediators in (A) healthy donors and LN, (B) non-proliferative (class I, II, V, or VI) and any proliferative (class III +/- V or class IV +/- V) (C) non-proliferative (class I, II, V, or VI) and pure proliferative (class III or IV), and (D) pure membranous (class V) and pure proliferative.
Figure 2. Soluble mediator profiles of lupus nephritis (LN) and proliferative LN. Volcano plots demonstrating the differential abundance of soluble mediators in (A) healthy donors and LN, (B) non-proliferative (class I, II, V, or VI) and any proliferative (class III +/- V or class IV +/- V) (C) non-proliferative (class I, II, V, or VI) and pure proliferative (class III or IV), and (D) pure membranous (class V) and pure proliferative.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1290007-3-ANY.jpg width=440 height=398.244028405423 border=0 style=border-style: none;>
Disclosures: A. Fava, Sanofi; C. Guthridge, None; J. Kheir, None; C. Wagner, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; J. Buyon, None; B. Diamond, None; t. (AMP) RA/SLE, None; J. Guthridge, None; J. James, Bristol-Myers Squibb(BMS), AstraZeneca, Novartis, Progentec Biosciences.
Background/Purpose: There is a pressing need to identify novel therapeutic approaches and noninvasive biomarkers in lupus nephritis (LN). In this study, we quantified serum soluble mediators in the large Accelerating Medicines Partnership (AMP) LN longitudinal cohort to identify novel biomarkers of histological features and treatment response and provide insights into the pathogenesis of LN.
Methods: SLE patients meeting ACR or SLICC criteria (n=268) undergoing a clinically indicated kidney biopsy with a urine protein/creatinine (UPCR) ≥0.5 were recruited for this study as part of the AMP. Serum samples were collected from patients at the time of diagnostic kidney biopsy and 3-, 6-, and 12-months post-biopsy and from 22 healthy donors (HD). Concentrations of 51 analytes, including cytokines, chemokines, and TNFR superfamily members, were analyzed by xMAP multiplex assays on the Bio-Rad BioPlex200® array system. TACE levels were determined by ELISA. Clinical response was determined at 12-months post-biopsy using the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study definitions in patients with a baseline UPCR >1.0 and International Society of Nephrology/Renal Pathology Society class III, IV, V, or combination thereof.
Results: LN patients demonstrated heterogeneity in serum soluble mediator concentrations (Fig 1). Most soluble mediators were elevated in LN patients compared to HD (Fig. 2A). Within LN, patients with proliferative LN (class III +/- V or class IV +/-V) displayed a distinct signature (Fig 2B). In particular, patients with pure proliferative LN (class III or IV) had higher serum levels of immune mediators, such as IFNβ and IL-1β, compared to nonproliferative LN (Fig 2C), including pure membranous (class V) LN (Fig 2D). In addition, several serum immune mediators correlated with intrarenal LN activity (NIH activity index), with syndecan-1 (CD138) and TNF-RII exhibiting the highest correlation (Fig 3A). In contrast, stem cell factor (SCF) and TNF-RI had the highest correlation with the chronicity index (Fig 2B). In proliferative LN, the concentrations of many immune mediators declined in treatment responders (not shown). For example, syndecan-1 and TNF-RII decreased in complete and partial responders but not in nonresponders (Fig 3C-D). In contrast, SCF levels remained relatively stable regardless of response status (Fig 3E).
Conclusion: Distinct histological features of LN are paralleled by specific signatures of circulating soluble mediators. Within LN, proliferative LN is associated with higher circulating levels of inflammatory cytokines, notably, type 1 IFNs. Furthermore, a decline in the titers of several immune mediators correlated with LN activity was associated with treatment response, suggesting a possible role in LN pathogenesis. These signatures and trajectories provide insight into LN pathogenesis, heterogeneity, and biomarker development.
 Figure 1. Soluble mediator heterogeneity in lupus nephritis (LN) patients. Heatmap of the soluble mediator concentrations in healthy donors (HD) and LN patients. International Society of Nephrology/Renal Pathology Society (ISN) class and proliferative versus non-proliferative LN are indicated by bars on the top of the heatmap.
Figure 1. Soluble mediator heterogeneity in lupus nephritis (LN) patients. Heatmap of the soluble mediator concentrations in healthy donors (HD) and LN patients. International Society of Nephrology/Renal Pathology Society (ISN) class and proliferative versus non-proliferative LN are indicated by bars on the top of the heatmap.  Figure 2. Soluble mediator profiles of lupus nephritis (LN) and proliferative LN. Volcano plots demonstrating the differential abundance of soluble mediators in (A) healthy donors and LN, (B) non-proliferative (class I, II, V, or VI) and any proliferative (class III +/- V or class IV +/- V) (C) non-proliferative (class I, II, V, or VI) and pure proliferative (class III or IV), and (D) pure membranous (class V) and pure proliferative.
Figure 2. Soluble mediator profiles of lupus nephritis (LN) and proliferative LN. Volcano plots demonstrating the differential abundance of soluble mediators in (A) healthy donors and LN, (B) non-proliferative (class I, II, V, or VI) and any proliferative (class III +/- V or class IV +/- V) (C) non-proliferative (class I, II, V, or VI) and pure proliferative (class III or IV), and (D) pure membranous (class V) and pure proliferative. <img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1290007-3-ANY.jpg width=440 height=398.244028405423 border=0 style=border-style: none;>
Figure 3. Serum biomarkers of histological activity, chronicity, and treatment response.
Volcano plots displaying Spearman correlations of the (A) NIH activity index and (B) NIH chronicity index with the serum concentrations of soluble mediators at the time of the diagnostic kidney biopsy. (C-E) Examples of trajectories. Soluble mediator concentrations were measured at the time of kidney biopsy (V0) and 3 (V1), 6 (V2), and 12 (V3) months post-kidney biopsy in patients with proliferative LN (class III +/-V or class IV +/- V). Treatment response was determined at 12 months, with complete response (CR) defined as urine protein//creatinine (UPCR) < 0.5, serum creatinine < 125% of baseline, and prednisone < 10mg/day. Partial response (PR) was defined by the same criteria as CR, except with a UPCR >0.5 with < 50% improvement from baseline. Patients who did not meet either requirement were classified as nonresponders (NR).
Volcano plots displaying Spearman correlations of the (A) NIH activity index and (B) NIH chronicity index with the serum concentrations of soluble mediators at the time of the diagnostic kidney biopsy. (C-E) Examples of trajectories. Soluble mediator concentrations were measured at the time of kidney biopsy (V0) and 3 (V1), 6 (V2), and 12 (V3) months post-kidney biopsy in patients with proliferative LN (class III +/-V or class IV +/- V). Treatment response was determined at 12 months, with complete response (CR) defined as urine protein//creatinine (UPCR) < 0.5, serum creatinine < 125% of baseline, and prednisone < 10mg/day. Partial response (PR) was defined by the same criteria as CR, except with a UPCR >0.5 with < 50% improvement from baseline. Patients who did not meet either requirement were classified as nonresponders (NR).
Disclosures: A. Fava, Sanofi; C. Guthridge, None; J. Kheir, None; C. Wagner, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; J. Buyon, None; B. Diamond, None; t. (AMP) RA/SLE, None; J. Guthridge, None; J. James, Bristol-Myers Squibb(BMS), AstraZeneca, Novartis, Progentec Biosciences.

