Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0929: Severe Low-Dose Methotrexate Toxicity in Elderly Patients with Inflammatory Rheumatic Diseases
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- MA
Martin Aringer, MD
University Medical Center and Faculty of Medicine TU Dresden
Dresden, Germany
Abstract Poster Presenter(s)
Cara Kumar1, Kristine Herrmann1 and Martin Aringer2, 1Division of Rheumatology, Department of Medicine III, University Medical Center and Faculty of Medicine TU Dresden, Dresden, Germany, 2University Medical Center and Faculty of Medicine TU Dresden, Dresden, Germany
Background/Purpose: While mostly remarkably safe, low dose methotrexate (MTX) occasionally causes life-threatening events. We analyzed all patients with rheumatic diseases and severe MTX toxicity between 2011 and 2020 for outcome, presentation, and associated risk factors.
Methods: We collected data on age, sex, diagnosis, dose and duration of MTX-treatment, concomitant medication, manifestations of toxicity, infections and outcome from the electronic patient files. Renal function, expressed as estimated glomerular filtration rate (eGFR), was assessed months before MTX toxicity had occurred, at admission, in the course of hospitalization, and at discharge. 150 patients with rheumatoid arthritis (RA) who were more than 70 years old were analyzed as a comparator group.
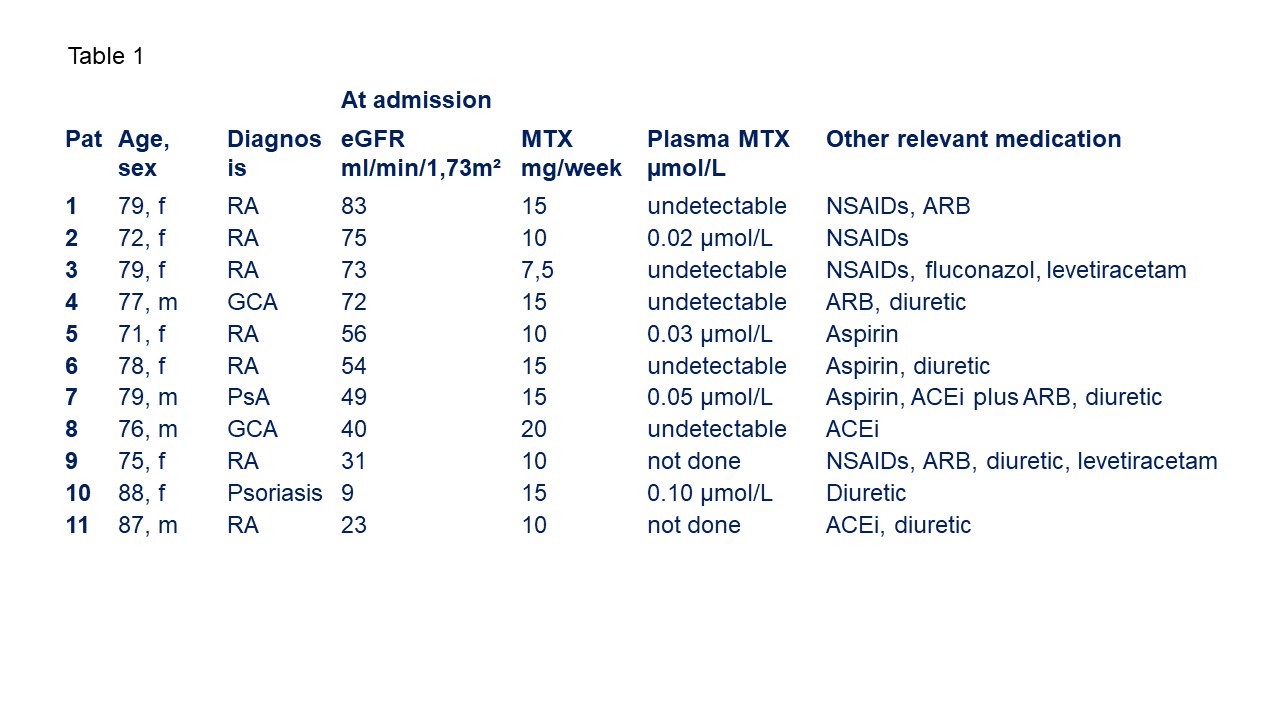
Results: 11 (7 female, 4 male) patients, aged 71 to 88 years, were admitted for severe MTX toxicity. 7 patients had RA, 2 each psoriasis/psoriatic arthritis and giant cell arteritis. At admission, all 11 patients had severe mucositis, 8 had pancytopenia, 10 patients presented with renal impairment and 6 with skin lesions. 5 patients had septicemia and 4 had pulmonary infiltrates. One patient succumbed to complications of septicemia; the other nine patients were discharged from the hospital after an average of 26 hospital days.
MTX had been newly initiated in two patients, but established a mean 5.8 years (3 months months - 20 years) in the remaining 8. The last prescribed MTX dose was 13.3±3.5 mg/week (mean ± SD), ranging from 7.5 to 20 mg/week. MTX could be detected in plasma of 4 out of 9 tested patients (0.02 to 0.10 µmol/L). Accidental overdosing was clinically suspected in only one patient. In addition to MTX, 4 patients took NSAIDs/coxibs, 4 angiotensin receptor blockers (ARBs), 2 ACE inhibitors (ACEi), and 5 diuretics. 2 patients had the combination of NSAIDs, angiotensin receptor or ACE inhibitors, and diuretics.
Of 9 patients with eGFR before the event were available, 5 (63%) had mild (60-89 mL/min/1,73 m²), 3 (38%) moderate renal insufficiency (30-59 mL/min/1,73m²). At admission, 4 patients had mild, 5 moderate renal insufficiency, one patient had an eGFR of 9 mL/min/1,73m².
The 150 RA patients on 14.3±4.9 mg MTX/week used ARB/ACEi and NSAIDs/coxibs in similar proportions, but less commonly diuretics (18/150) than those with severe toxicity (5/11, p=0.01 Fisher's exact test). Their eGFR was 70 [8-90] mL/min/1,73m², higher than in the patients with toxicity (median 61 [32-77] mL/min/1,73m², p=0.0171. 8 (5%) had normal renal function, 105 (70%) mild, 35 (23%) moderate insufficiency and one an eGFR of 19 mL/min/1,73m².
Conclusion: Life-threatening MTX complications occurred only in patients past 70 years of age and with previous mild to moderate impairment of renal function. While measurable MTX levels may suggest accidental overdosing in 4 patients, acute renal failure, possibly in part remitted by the time of admission, was the most likely cause in the majority of patients. Compared to an RA population of similar age, renal function was slightly worse on a group level, and the use of diuretics was more common in those with severe toxicity.
Disclosures: C. Kumar, None; K. Herrmann, None; M. Aringer, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Merck/MSD, Novartis, Pfizer, Roche, Galapagos, Otsuka.
Background/Purpose: While mostly remarkably safe, low dose methotrexate (MTX) occasionally causes life-threatening events. We analyzed all patients with rheumatic diseases and severe MTX toxicity between 2011 and 2020 for outcome, presentation, and associated risk factors.
Methods: We collected data on age, sex, diagnosis, dose and duration of MTX-treatment, concomitant medication, manifestations of toxicity, infections and outcome from the electronic patient files. Renal function, expressed as estimated glomerular filtration rate (eGFR), was assessed months before MTX toxicity had occurred, at admission, in the course of hospitalization, and at discharge. 150 patients with rheumatoid arthritis (RA) who were more than 70 years old were analyzed as a comparator group.
Results: 11 (7 female, 4 male) patients, aged 71 to 88 years, were admitted for severe MTX toxicity. 7 patients had RA, 2 each psoriasis/psoriatic arthritis and giant cell arteritis. At admission, all 11 patients had severe mucositis, 8 had pancytopenia, 10 patients presented with renal impairment and 6 with skin lesions. 5 patients had septicemia and 4 had pulmonary infiltrates. One patient succumbed to complications of septicemia; the other nine patients were discharged from the hospital after an average of 26 hospital days.
MTX had been newly initiated in two patients, but established a mean 5.8 years (3 months months - 20 years) in the remaining 8. The last prescribed MTX dose was 13.3±3.5 mg/week (mean ± SD), ranging from 7.5 to 20 mg/week. MTX could be detected in plasma of 4 out of 9 tested patients (0.02 to 0.10 µmol/L). Accidental overdosing was clinically suspected in only one patient. In addition to MTX, 4 patients took NSAIDs/coxibs, 4 angiotensin receptor blockers (ARBs), 2 ACE inhibitors (ACEi), and 5 diuretics. 2 patients had the combination of NSAIDs, angiotensin receptor or ACE inhibitors, and diuretics.
Of 9 patients with eGFR before the event were available, 5 (63%) had mild (60-89 mL/min/1,73 m²), 3 (38%) moderate renal insufficiency (30-59 mL/min/1,73m²). At admission, 4 patients had mild, 5 moderate renal insufficiency, one patient had an eGFR of 9 mL/min/1,73m².
The 150 RA patients on 14.3±4.9 mg MTX/week used ARB/ACEi and NSAIDs/coxibs in similar proportions, but less commonly diuretics (18/150) than those with severe toxicity (5/11, p=0.01 Fisher's exact test). Their eGFR was 70 [8-90] mL/min/1,73m², higher than in the patients with toxicity (median 61 [32-77] mL/min/1,73m², p=0.0171. 8 (5%) had normal renal function, 105 (70%) mild, 35 (23%) moderate insufficiency and one an eGFR of 19 mL/min/1,73m².
Conclusion: Life-threatening MTX complications occurred only in patients past 70 years of age and with previous mild to moderate impairment of renal function. While measurable MTX levels may suggest accidental overdosing in 4 patients, acute renal failure, possibly in part remitted by the time of admission, was the most likely cause in the majority of patients. Compared to an RA population of similar age, renal function was slightly worse on a group level, and the use of diuretics was more common in those with severe toxicity.

Disclosures: C. Kumar, None; K. Herrmann, None; M. Aringer, AbbVie/Abbott, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Merck/MSD, Novartis, Pfizer, Roche, Galapagos, Otsuka.

