Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0913–0938) RA – Treatment Poster II
0916: Should RA Patients with Controlled Disease Taper Methotrexate from Targeted Therapy or Continue It? Risk Differences in Sustaining Remission from a Systematic Review and Meta-analysis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- CM
Charis Meng, MD
Hospital for Special Surgery
New York, NY, United States
Abstract Poster Presenter(s)
Charis Meng, Diviya Rajesh, Bridget Jivanelli, Deanna Jannat-Khah, DrPH, MSPH and Vivian Bykerk, Hospital for Special Surgery, New York, NY
Background/Purpose: Patients with RA often struggle with side effects of methotrexate (MTX). ACR guidelines conditionally recommend the tapering of MTX before tapering biologic (b)DMARDs, but acknowledge there is an absence of direct evidence. Prior reviews have focused on the tapering of MTX when used with TNF-i only1. There have been no updated reviews addressing MTX tapering when used with other targeted therapies such as IL6-i or JAK-i. Physicians need a clinically useful quantification of risk of losing remission to guide their patients in deciding whether to taper MTX when combined with targeted therapy. Our purpose was to determine the risk of being unable to sustain RA remission when tapering MTX from combined treatment with targeted therapy (bDMARDs or JAK-i).
Methods: A systematic literature search using MeSH terms and keywords was conducted in Medline, Embase and Cochrane Library for studies in RA reporting remission outcomes after tapering MTX from combined treatment with targeted therapies. We used random effects models, and calculated the relative risk, risk differences and heterogeneity. Forest plots were created.
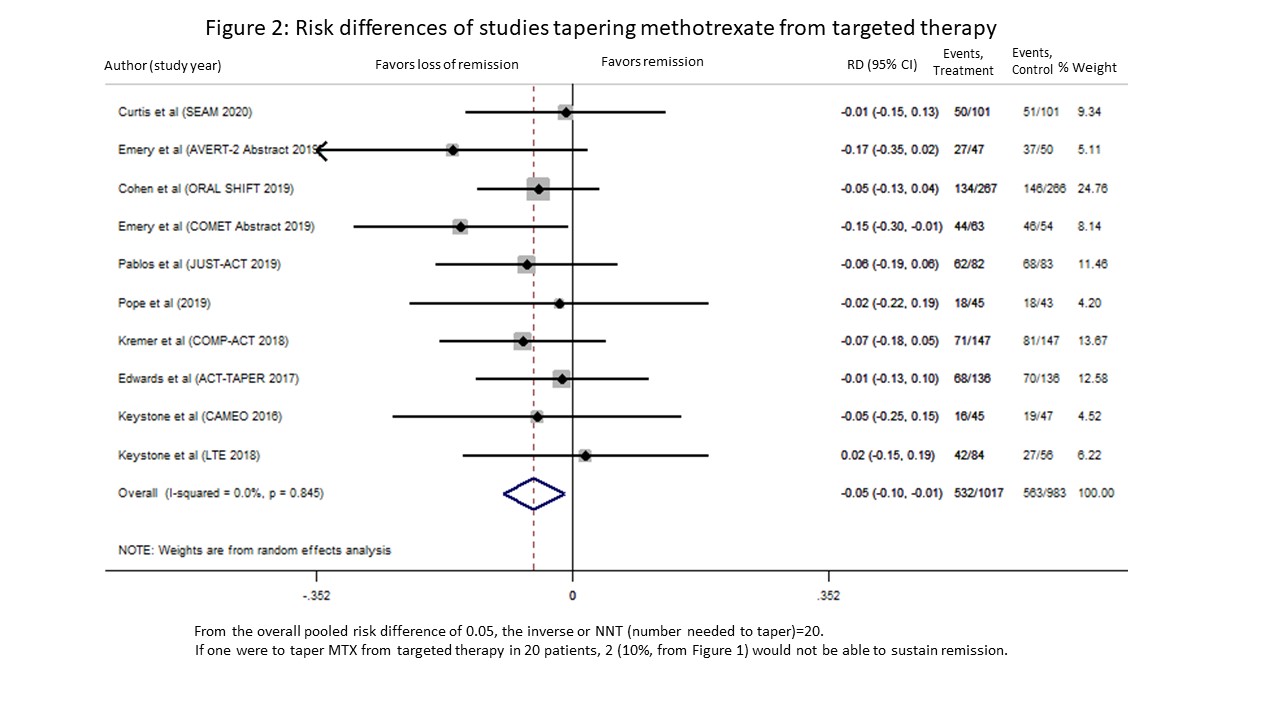
Results: Our search identified 5762 citations. After removal of duplicates and screening title/abstract using the COVIDENCE platform, 504 full-text articles were reviewed. Of these, 10 studies met our inclusion criteria of tapering MTX from targeted therapy; in 3 studies MTX was tapered from etanercept, 3 from tocilizumab, and 1 each from tofacitinib, certolizumab pegol, adalimumab and abatacept. Nine were RCTs, and one was observational (Table 1). Three trials studied early RA (1-9 months). The MTX tapering strategy was gradual in 2 and rapid in 8 studies. Follow-up ranged from 3 to 18 months in the RCTs. Our meta-analysis of 2000 RA participants showed those who tapered MTX from targeted therapy had a 10% reduction in ability to sustain remission, an overall pooled RR 0.90 (95% CI 0.84, 0.97) (Figure 1). There was no heterogeneity, (I2=0.0%, p=0.938). Risk differences were calculated with an overall pooled RD of -0.05 (95% CI -0.10, -0.01) (Figure 2). Using the pooled estimate, if one were to taper MTX from a targeted therapy in 20 patients, 2 (10%) patients would not be able to sustain remission.
Conclusion: This is the first systematic review and meta-analysis assessing if MTX can be tapered from advanced therapies that target different pathways in controlled RA and indicates patients may do so, but risk a 10% reduction in ability to sustain remission, for up to 18 months. The overall risk difference between tapering MTX from targeted therapy or not tapering was low. This review may help inform ACR guidelines, and discussions with patients with controlled disease on any of a range of targeted therapies and MTX, who struggle with MTX-related adverse effects and wish to taper it. Longer follow-up studies including radiographic, functional and patient-reported outcomes are still needed. The risk of disease worsening should be discussed with the patient with careful follow-up and prompt re-treatment if RA disease activity worsens.
References:
1. Subesinghe S, Scott IC. Expert Rev Clin Pharmacol 2015;8:751-60.
.jpg)
.jpg)
Disclosures: C. Meng, None; D. Rajesh, None; B. Jivanelli, None; D. Jannat-Khah, DrPH, MSPH, Cytodyn, AstraZeneca, Walgreens; V. Bykerk, Janssen, Bristol Myers Squibb, Crossbridge, Pfizer, Sanofi Aventis, Brainstorm Therapeutics, Amgen, UCB, Gilead, Genzyme Corporation, Regeneron.
Background/Purpose: Patients with RA often struggle with side effects of methotrexate (MTX). ACR guidelines conditionally recommend the tapering of MTX before tapering biologic (b)DMARDs, but acknowledge there is an absence of direct evidence. Prior reviews have focused on the tapering of MTX when used with TNF-i only1. There have been no updated reviews addressing MTX tapering when used with other targeted therapies such as IL6-i or JAK-i. Physicians need a clinically useful quantification of risk of losing remission to guide their patients in deciding whether to taper MTX when combined with targeted therapy. Our purpose was to determine the risk of being unable to sustain RA remission when tapering MTX from combined treatment with targeted therapy (bDMARDs or JAK-i).
Methods: A systematic literature search using MeSH terms and keywords was conducted in Medline, Embase and Cochrane Library for studies in RA reporting remission outcomes after tapering MTX from combined treatment with targeted therapies. We used random effects models, and calculated the relative risk, risk differences and heterogeneity. Forest plots were created.
Results: Our search identified 5762 citations. After removal of duplicates and screening title/abstract using the COVIDENCE platform, 504 full-text articles were reviewed. Of these, 10 studies met our inclusion criteria of tapering MTX from targeted therapy; in 3 studies MTX was tapered from etanercept, 3 from tocilizumab, and 1 each from tofacitinib, certolizumab pegol, adalimumab and abatacept. Nine were RCTs, and one was observational (Table 1). Three trials studied early RA (1-9 months). The MTX tapering strategy was gradual in 2 and rapid in 8 studies. Follow-up ranged from 3 to 18 months in the RCTs. Our meta-analysis of 2000 RA participants showed those who tapered MTX from targeted therapy had a 10% reduction in ability to sustain remission, an overall pooled RR 0.90 (95% CI 0.84, 0.97) (Figure 1). There was no heterogeneity, (I2=0.0%, p=0.938). Risk differences were calculated with an overall pooled RD of -0.05 (95% CI -0.10, -0.01) (Figure 2). Using the pooled estimate, if one were to taper MTX from a targeted therapy in 20 patients, 2 (10%) patients would not be able to sustain remission.
Conclusion: This is the first systematic review and meta-analysis assessing if MTX can be tapered from advanced therapies that target different pathways in controlled RA and indicates patients may do so, but risk a 10% reduction in ability to sustain remission, for up to 18 months. The overall risk difference between tapering MTX from targeted therapy or not tapering was low. This review may help inform ACR guidelines, and discussions with patients with controlled disease on any of a range of targeted therapies and MTX, who struggle with MTX-related adverse effects and wish to taper it. Longer follow-up studies including radiographic, functional and patient-reported outcomes are still needed. The risk of disease worsening should be discussed with the patient with careful follow-up and prompt re-treatment if RA disease activity worsens.
References:
1. Subesinghe S, Scott IC. Expert Rev Clin Pharmacol 2015;8:751-60.
.jpg)
.jpg)

Disclosures: C. Meng, None; D. Rajesh, None; B. Jivanelli, None; D. Jannat-Khah, DrPH, MSPH, Cytodyn, AstraZeneca, Walgreens; V. Bykerk, Janssen, Bristol Myers Squibb, Crossbridge, Pfizer, Sanofi Aventis, Brainstorm Therapeutics, Amgen, UCB, Gilead, Genzyme Corporation, Regeneron.

