Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0974–1003) SLE – Treatment Poster II
0996: Tapering of Corticosteroids or Immunosuppressive Therapy in Stable SLE: A Comparison of Complete Remission, Clinical Remission and Lupus Low Disease Activity State in Protection Against Flares
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- JC
Jiacai Cho, MBBS, MRCP, MMed
National University Hospital
Singapore, Singapore
Abstract Poster Presenter(s)
Jiacai Cho1, liang shen2, Molla Huq3, Rangi Kandane-Rathnayake4, Vera Golder4, Worawit Louthrenoo5, Yi-Hsin Chen6, Laniyati Hamijoyo7, Luo Shue Fen8, Yeong-Jian Wu8, Leonid Zamora9, Zhouli Zhang10, An Yuan11, Sargunan Sockalingam12, Yasuhiro Katsumata13, Masayoshi Harigai13, Yanjie Hao3, Zhanguo Li14, Duminda Basnayake15, Madelynn Chan16, Jun Kikuchi17, Tsutomu Takeuchi18, Sang-Cheol Bae19, Fiona Goldblatt20, Shereen Oon21, Sean O'Neill22, Kathy Gibson22, Kristine Ng23, Hui Nee Annie Law24, Nicole Tugnet25, Sunil Kumar26, Cherica Tee27, Michael Tee27, Naoaki Ohkubo28, Yoshiya Tanaka28, Sandra Navarra29, Chak Sing30, Alberta Hoi31, Eric Morand32, Mandana Nikpour33 and Aisha Lateef34, 1National University Health System (NUHS), Singapore, Singapore, 2National University of Singapore, Singapore, Singapore, 3The University of Melbourne, Melbourne, Australia, 4Monash University, Clayton, Australia, 5Chiang Mai University, Chiang Mai, Thailand, 6Taichung Veterans General Hospital, Taichung, Taiwan, 7Hasan Sadikin Hospital, Jakarta Selatan, Indonesia, 8Chang Gung Memorial Hospital, Taoyuan, Taiwan, 9University of Santo Tomas Hospital, Manila, Philippines, 10Peking University First Hospital, Beijing, China, 11Peking University Health Science Center, Beijing, China, 12University of Malaya, Kuala Lumpur, Malaysia, 13Division of Rheumatology, Department of Internal Medicine, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan, 14People's Hospital, Peking University Health Science Center, Beijing, China, 15Teaching Hospital Kandy, Kandy, Sri Lanka, 16Tan Tock Seng Hospital, Singapore, Singapore, 17Keio University, Tokyo, Japan, 18Keio University and Saitama Medical University, Tokyo, Japan, 19Hanyang University Medical Center, Seoul, Republic of Korea, 20Royal Adelaide Hospital and Flinders Medical Centre, Adelaide, Australia, 21St Vincent's Hospital, Fitzroy, Australia, 22Liverpool Hospital, Sydney, Australia, 23North Shore Hospital, Auckland, New Zealand, 24Department of Rheumatology and Immunology, Singapore General Hospital, Singapore, Singapore, 25Greenlane Clinical Centre, Auckland, New Zealand, 26Middlemore Hospital, Auckland, New Zealand, 27University of the Philippines, Quezon City, Philippines, 28University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 29University of Santo Tomas, Manila, Philippines, 30The University of Hong Kong, Pok Fu Lam, Hong Kong, 31Monash Health, Melbourne, Australia, 32Monash University, Victoria; Department of Rheumatology, Monash Health, Melbourne, Australia, 33The University of Melbourne at St. Vincent's Hospital Melbourne, Melbourne, Australia, 34National University Hospital, Singapore, Singapore
Background/Purpose: Proposed targets of SLE treatment include lupus low disease activity state (LLDAS), clinical remission and complete remission. Whether treatment can be tapered after achieving these targets and whether tapering will be safer in deeper states of remission compared to LLDAS are unknown.
Methods: We collected data from SLE patients (ACR/SLICC criteria) prospectively between 2013 to 2020 from 13 Asia-pacific countries. Disease activity was captured at each visit using SLEDAI-2K. We defined flares using SELEA-SLEDAI Flare Index. Tapering was defined as any decrease in dose of corticosteroids or immunosuppressive therapy (MMF, CSA, AZA, LEF, MTX or tacrolimus). We studied all visits that had achieved target, including LLDAS1, 2021 DORIS definition of clinical remission (clinical SLEDAI=0, PGA< 0.5, prednisolone ≤5mg/day)2 or DORIS complete remission on therapy (SLEDAI-2K=0, PGA< 0.5, prednisolone ≤5mg/day)3. Using multivariable generalized estimating equations, we compared flares at the subsequent visit, after drug tapering versus continuing the same therapy.
We then further analyzed tapering that occurred across visits by grouping consecutive visits into a single “tapering attempt”. A tapering attempt began with any taper of corticosteroids or immunosuppressive therapy at one of the target SLE states and ended at a visit when the dose of corticosteroid or immunosuppressive agent was increased. We used multivariable COX-proportional hazards to analyze the time to first flare, comparing attempts which had begun in LLDAS, clinical remission and complete remission.
Results: We included 3002 patients and 14808 patient-visits that had achieved one of the target SLE states (LLDAS, clinical remission or complete remission). Tapering occurred in 3521 visits (23.8%) and 11287 (76.2%) continued on the same therapy. 1679 flares (12.8%) were captured at the next visit. Tapering was associated with 1.24 higher odds of flare compared to continuing same therapy (p< 0.01). Other statistically significant associations included use of antimalarials, country, adjusted mean SLEDAI, disease duration and cumulative LLDAS/remission duration before taper (Table 1).
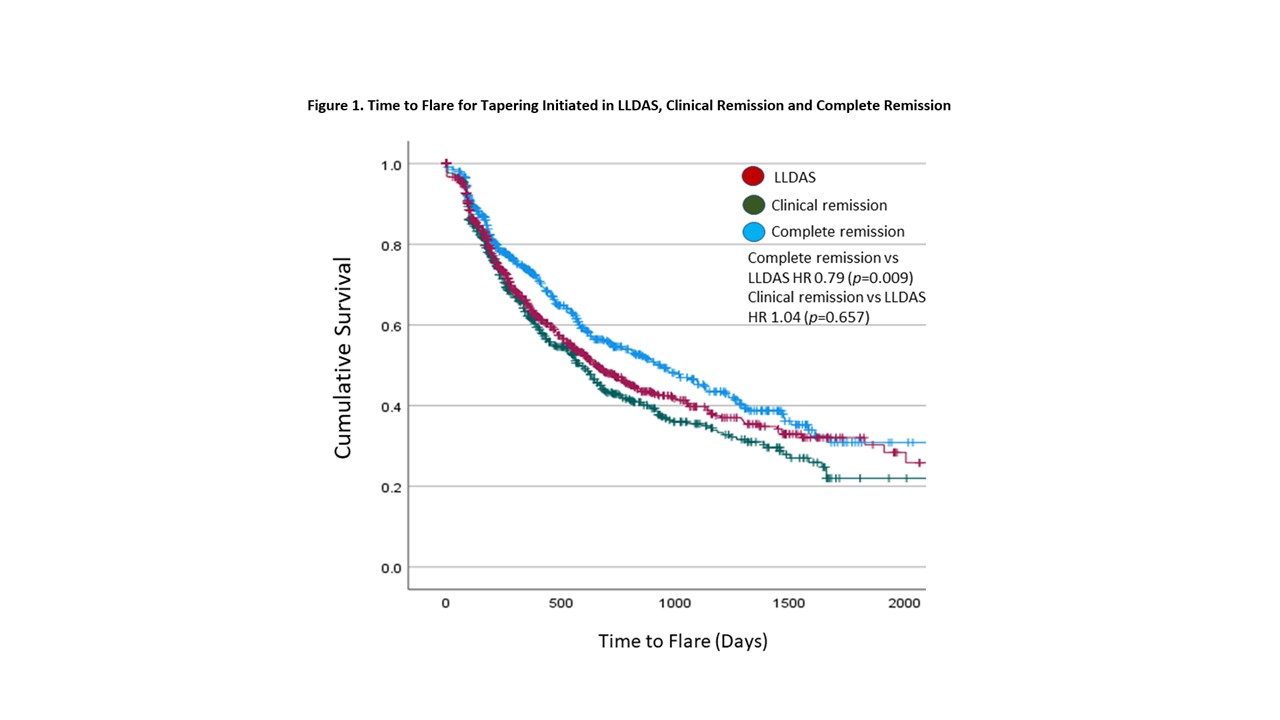
We recorded 2095 tapering attempts, of which 41.1%, 28.4% and 30.5% were initiated in LLDAS, clinical remission and complete remission respectively. The median (IQR) time to flare was 238 (33-443) days. Tapering attempts that were initiated in complete remission had a significantly longer time to first flare compared to attempts that were initiated in clinical remission or LLDAS (HR 0.79, p=0.009, Figure 1). Other significant variables associated with longer time to flare included country, duration of LLDAS/remission more than 30 days before taper, older age, shorter disease duration and lower adjusted mean SLEDAI (Table 2).
Conclusion: Tapering of corticosteroids or immunosuppressive therapy in stable SLE was associated with increased flares. Attaining complete remission was more protective against flares upon drug tapering as compared to attaining LLDAS or clinical remission. Drug tapering should be carefully considered in stable SLE patients irrespective with LLDAS or either type of remission.
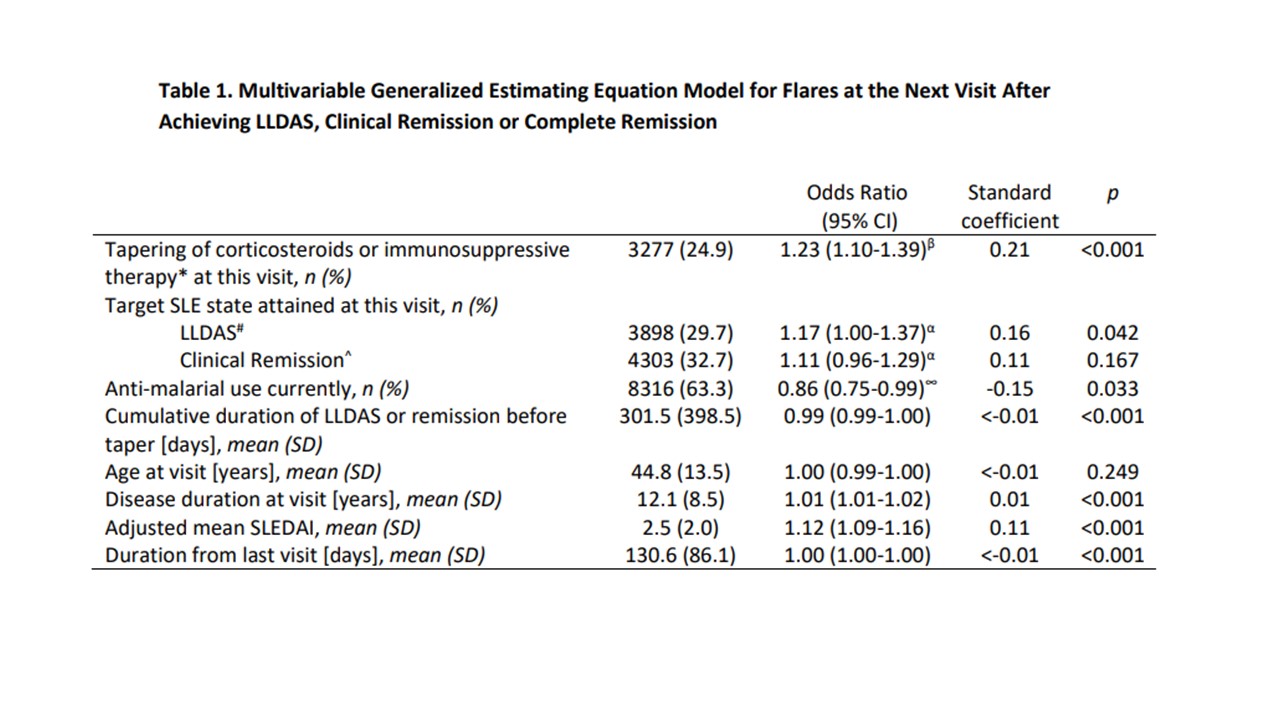 * Includes MMF, CSA, AZA, LEF, MTX or tacrolimus
* Includes MMF, CSA, AZA, LEF, MTX or tacrolimus
β Compared to continuation of therapy without tapering
# LLDAS; Lupus Low Disease Activity State; SLEDAI-2K≤4, PGA < 0.5, prednisolone ≤7.5mg/day)
^Clinical remission; clinical SLEDAI=0, PGA < 0.5, prednisolone ≤5mg/day
α Compared to Complete remission; SLEDAI-2K=0, PGA < 0.5, prednisolone ≤5mg/day
∞ Compared to non-use of anti-malarials at current visit
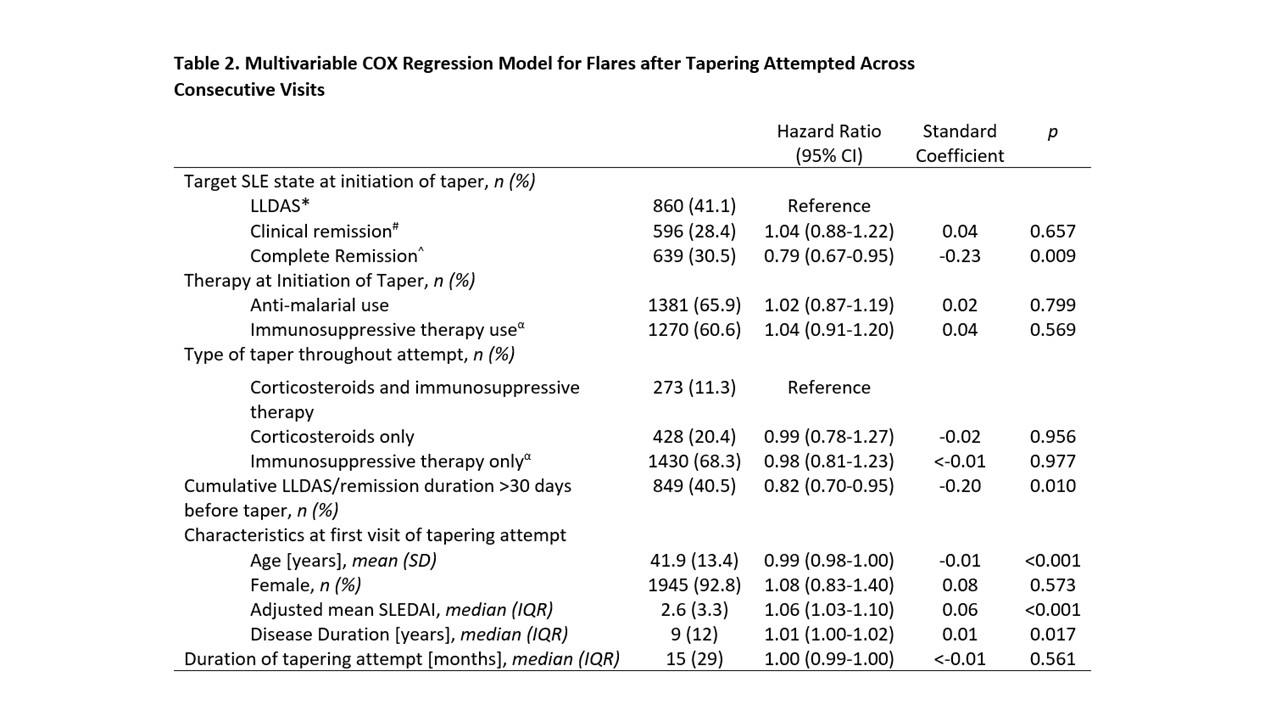 * LLDAS; Lupus Low Disease Activity State; SLEDAI-2K≤4, PGA < 0.5, prednisolone ≤7.5mg/day)
* LLDAS; Lupus Low Disease Activity State; SLEDAI-2K≤4, PGA < 0.5, prednisolone ≤7.5mg/day)
# Clinical remission; clinical SLEDAI=0, PGA < 0.5, prednisolone ≤5mg/day
^ Complete remission; SLEDAI-2K=0, PGA < 0.5, prednisolone ≤5mg/day
α Includes MMF, CSA, AZA, LEF, MTX or tacrolimus
Disclosures: J. Cho, None; l. shen, None; M. Huq, None; R. Kandane-Rathnayake, None; V. Golder, None; W. Louthrenoo, None; Y. Chen, None; L. Hamijoyo, None; L. Fen, None; Y. Wu, None; L. Zamora, None; Z. Zhang, None; A. Yuan, None; S. Sockalingam, None; Y. Katsumata, GlaxoSmithKline K.K., AstraZeneca K.K., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., Asahi Kasei Pharma, Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc.; M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan; Y. Hao, None; Z. Li, None; D. Basnayake, None; M. Chan, None; J. Kikuchi, None; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; S. Bae, None; F. Goldblatt, None; S. Oon, None; S. O'Neill, None; K. Gibson, Eli Lilly Pty Ltd; K. Ng, None; H. Law, None; N. Tugnet, None; S. Kumar, None; C. Tee, None; M. Tee, None; N. Ohkubo, None; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; S. Navarra, Biogen, Astellas, Janssen, Novartis, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline (GSK); C. Sing, None; A. Hoi, None; E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA; M. Nikpour, Janssen, AstraZeneca, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Bristol-Myers Squibb(BMS); A. Lateef, None.
Background/Purpose: Proposed targets of SLE treatment include lupus low disease activity state (LLDAS), clinical remission and complete remission. Whether treatment can be tapered after achieving these targets and whether tapering will be safer in deeper states of remission compared to LLDAS are unknown.
Methods: We collected data from SLE patients (ACR/SLICC criteria) prospectively between 2013 to 2020 from 13 Asia-pacific countries. Disease activity was captured at each visit using SLEDAI-2K. We defined flares using SELEA-SLEDAI Flare Index. Tapering was defined as any decrease in dose of corticosteroids or immunosuppressive therapy (MMF, CSA, AZA, LEF, MTX or tacrolimus). We studied all visits that had achieved target, including LLDAS1, 2021 DORIS definition of clinical remission (clinical SLEDAI=0, PGA< 0.5, prednisolone ≤5mg/day)2 or DORIS complete remission on therapy (SLEDAI-2K=0, PGA< 0.5, prednisolone ≤5mg/day)3. Using multivariable generalized estimating equations, we compared flares at the subsequent visit, after drug tapering versus continuing the same therapy.
We then further analyzed tapering that occurred across visits by grouping consecutive visits into a single “tapering attempt”. A tapering attempt began with any taper of corticosteroids or immunosuppressive therapy at one of the target SLE states and ended at a visit when the dose of corticosteroid or immunosuppressive agent was increased. We used multivariable COX-proportional hazards to analyze the time to first flare, comparing attempts which had begun in LLDAS, clinical remission and complete remission.
Results: We included 3002 patients and 14808 patient-visits that had achieved one of the target SLE states (LLDAS, clinical remission or complete remission). Tapering occurred in 3521 visits (23.8%) and 11287 (76.2%) continued on the same therapy. 1679 flares (12.8%) were captured at the next visit. Tapering was associated with 1.24 higher odds of flare compared to continuing same therapy (p< 0.01). Other statistically significant associations included use of antimalarials, country, adjusted mean SLEDAI, disease duration and cumulative LLDAS/remission duration before taper (Table 1).
We recorded 2095 tapering attempts, of which 41.1%, 28.4% and 30.5% were initiated in LLDAS, clinical remission and complete remission respectively. The median (IQR) time to flare was 238 (33-443) days. Tapering attempts that were initiated in complete remission had a significantly longer time to first flare compared to attempts that were initiated in clinical remission or LLDAS (HR 0.79, p=0.009, Figure 1). Other significant variables associated with longer time to flare included country, duration of LLDAS/remission more than 30 days before taper, older age, shorter disease duration and lower adjusted mean SLEDAI (Table 2).
Conclusion: Tapering of corticosteroids or immunosuppressive therapy in stable SLE was associated with increased flares. Attaining complete remission was more protective against flares upon drug tapering as compared to attaining LLDAS or clinical remission. Drug tapering should be carefully considered in stable SLE patients irrespective with LLDAS or either type of remission.
 * Includes MMF, CSA, AZA, LEF, MTX or tacrolimus
* Includes MMF, CSA, AZA, LEF, MTX or tacrolimusβ Compared to continuation of therapy without tapering
# LLDAS; Lupus Low Disease Activity State; SLEDAI-2K≤4, PGA < 0.5, prednisolone ≤7.5mg/day)
^Clinical remission; clinical SLEDAI=0, PGA < 0.5, prednisolone ≤5mg/day
α Compared to Complete remission; SLEDAI-2K=0, PGA < 0.5, prednisolone ≤5mg/day
∞ Compared to non-use of anti-malarials at current visit
 * LLDAS; Lupus Low Disease Activity State; SLEDAI-2K≤4, PGA < 0.5, prednisolone ≤7.5mg/day)
* LLDAS; Lupus Low Disease Activity State; SLEDAI-2K≤4, PGA < 0.5, prednisolone ≤7.5mg/day)# Clinical remission; clinical SLEDAI=0, PGA < 0.5, prednisolone ≤5mg/day
^ Complete remission; SLEDAI-2K=0, PGA < 0.5, prednisolone ≤5mg/day
α Includes MMF, CSA, AZA, LEF, MTX or tacrolimus

Disclosures: J. Cho, None; l. shen, None; M. Huq, None; R. Kandane-Rathnayake, None; V. Golder, None; W. Louthrenoo, None; Y. Chen, None; L. Hamijoyo, None; L. Fen, None; Y. Wu, None; L. Zamora, None; Z. Zhang, None; A. Yuan, None; S. Sockalingam, None; Y. Katsumata, GlaxoSmithKline K.K., AstraZeneca K.K., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., Asahi Kasei Pharma, Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc.; M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan; Y. Hao, None; Z. Li, None; D. Basnayake, None; M. Chan, None; J. Kikuchi, None; T. Takeuchi, Astellas Pharma, Eli Lilly Japan, Gilead Sciences, AbbVie, Eisai Co., Ltd, Pfizer Japan Inc., Asahi Kasei, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, UCB Japan, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Novartis, Sanofi; S. Bae, None; F. Goldblatt, None; S. Oon, None; S. O'Neill, None; K. Gibson, Eli Lilly Pty Ltd; K. Ng, None; H. Law, None; N. Tugnet, None; S. Kumar, None; C. Tee, None; M. Tee, None; N. Ohkubo, None; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; S. Navarra, Biogen, Astellas, Janssen, Novartis, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline (GSK); C. Sing, None; A. Hoi, None; E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA; M. Nikpour, Janssen, AstraZeneca, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Bristol-Myers Squibb(BMS); A. Lateef, None.

