Back
Poster Session B
Epidemiology, health policy and outcomes
Session: (0724–0751) Health Services Research Poster II
0746: Telemedicine for Follow-up of Lupus Nephritis in the Covid-19 Outbreak: A Pragmatic Randomized Controlled Trial
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- HS
Ho So, MD
The Chinese University of Hong Kong
Hong Kong, Hong Kong, China
Abstract Poster Presenter(s)
Ho SO1, Evelyn Chow2, Isaac Cheng1, Xerox Lau2, Tena Li1, Cheuk Chun Szeto2 and Lai-shan Tam1, 1The Chinese University of Hong Kong, Hong Kong, China, 2The Chinese University of Hong Kong, Hong Kong, Hong Kong
Background/Purpose: During the pandemic, vulnerable patients such as those with lupus nephritis (LN) faced the difficult choice between COVID-19 infection risk during a clinic visit and postponing the needed care. An option would be to adopt telemedicine (TM). Despite being widely adopted, the evidence supporting the use of TM in rheumatology has been limited. Thus, we conducted a pragmatic trial comparing Telemedicine and standard in-person follow-up (FU) in patients with LN ("Tele-LN"). We primarily aimed to evaluate the effectiveness to maintain disease activity control using TM compared to conventional in-person outpatient FU. The secondary objectives were to compare the patient reported outcomes, safety and cost-of-illness between the 2 modes of health care delivery.
Methods: This was a 1-year, randomized controlled trial conducted at a regional hospital in Hong Kong. From 5/2020, patients with a diagnosis of SLE according to the 2019 EULAR/ACR classification criteria followed up at the LN clinic were invited to participate. Patients needed to possess the technology for conducting a TM visit. Participants were randomized 1:1 to either TM (TM group) or standard FU (SF group). Patients randomized to receive TM FU were scheduled for real-time video consultations. Patients in the SF group received standard in-person outpatient care. An in-person clinic consultation could be arranged as requested by the patients or clinicians. Similarly, a TM consultation could be arranged as required.
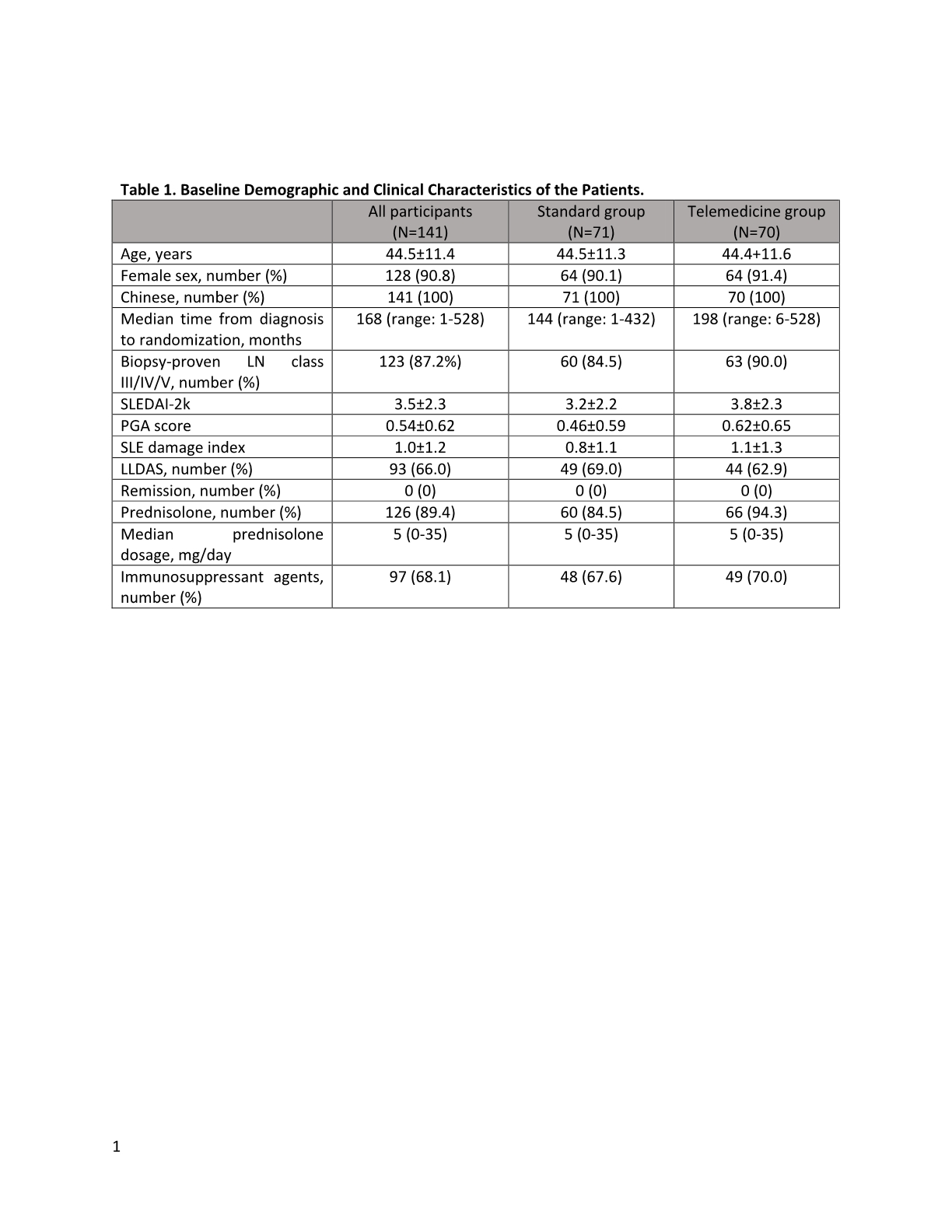
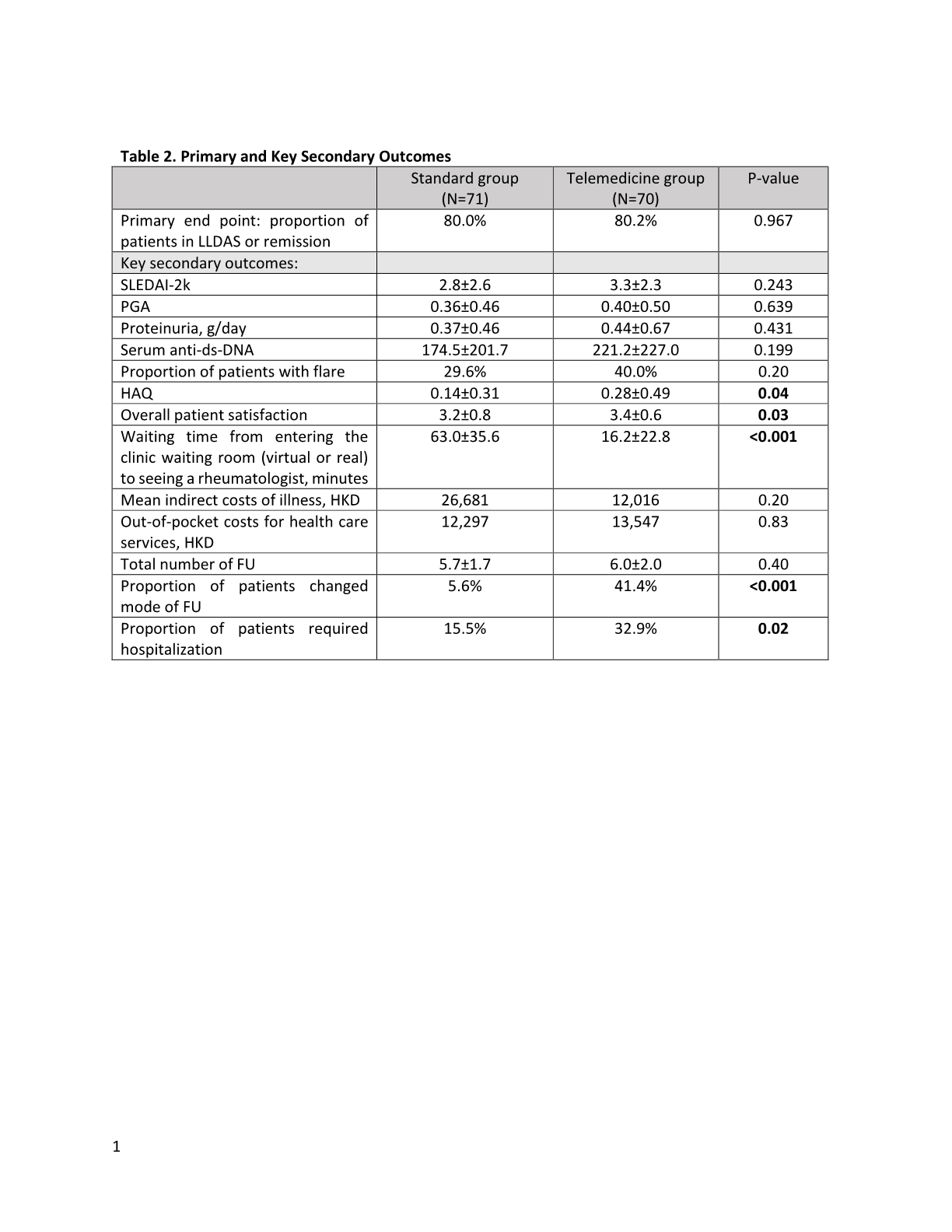
Results: At the end of the study (12/2021), 70 patients in the TM group and 71 patients in the SF completed one-year FU (Figure 1). There were no baseline differences between the 2 groups Table 1. The results with respect to the primary and major secondary end points are provided in Table 2. At 1 year, similar proportion of patients in the TM group and SF group were in lupus low disease activity state (LLDAS) or remission. SLE disease activity indices including SLEDAI-2k, PGA, proteinuria amount and serum anti-ds-DNA level remained similar between the 2 groups. There were no differences in the SF-36, lupusQoL and HADS scores between the 2 groups except a slightly higher HAQ in the TM group at the end of the study. The overall patient satisfaction score was higher in the TM group. At the end of the study, 67.9% of the overall participants agreed to use TM as a mode of future FU. Although the mean indirect costs of illness were numerically higher in the SF group, the out-of-pocket costs for health care services other than those provided by the public sector were similar between the two groups. The total number of FU was similar in the 2 groups. However, significantly more patients in the TM group requested change mode of FU. The proportion of patients requiring hospitalization was also higher in the TM group. Not being in LLDAS at baseline was the independent predictor of hospitalization (OR 3.4, 95%CI 1.20-9.65). None of the participants has been tested positive for COVID-19 throughout the study.
Conclusion: The results of the study suggest that TM delivered care could help maintaining disease control and psychosocial wellbeing during the pandemic, but it needs to be complemented by physical visits as required, particularly in those with unstable disease.
.jpg)
Disclosures: H. SO, None; E. Chow, None; I. Cheng, None; X. Lau, None; T. Li, None; C. Szeto, None; L. Tam, AbbVie, Amgen, Boehringer-Ingelheim, Eli Lilly, GlaxoSmithKlein(GSK), Janssen, Novartis, Pfizer, Sanofi, AstraZeneca.
Background/Purpose: During the pandemic, vulnerable patients such as those with lupus nephritis (LN) faced the difficult choice between COVID-19 infection risk during a clinic visit and postponing the needed care. An option would be to adopt telemedicine (TM). Despite being widely adopted, the evidence supporting the use of TM in rheumatology has been limited. Thus, we conducted a pragmatic trial comparing Telemedicine and standard in-person follow-up (FU) in patients with LN ("Tele-LN"). We primarily aimed to evaluate the effectiveness to maintain disease activity control using TM compared to conventional in-person outpatient FU. The secondary objectives were to compare the patient reported outcomes, safety and cost-of-illness between the 2 modes of health care delivery.
Methods: This was a 1-year, randomized controlled trial conducted at a regional hospital in Hong Kong. From 5/2020, patients with a diagnosis of SLE according to the 2019 EULAR/ACR classification criteria followed up at the LN clinic were invited to participate. Patients needed to possess the technology for conducting a TM visit. Participants were randomized 1:1 to either TM (TM group) or standard FU (SF group). Patients randomized to receive TM FU were scheduled for real-time video consultations. Patients in the SF group received standard in-person outpatient care. An in-person clinic consultation could be arranged as requested by the patients or clinicians. Similarly, a TM consultation could be arranged as required.
Results: At the end of the study (12/2021), 70 patients in the TM group and 71 patients in the SF completed one-year FU (Figure 1). There were no baseline differences between the 2 groups Table 1. The results with respect to the primary and major secondary end points are provided in Table 2. At 1 year, similar proportion of patients in the TM group and SF group were in lupus low disease activity state (LLDAS) or remission. SLE disease activity indices including SLEDAI-2k, PGA, proteinuria amount and serum anti-ds-DNA level remained similar between the 2 groups. There were no differences in the SF-36, lupusQoL and HADS scores between the 2 groups except a slightly higher HAQ in the TM group at the end of the study. The overall patient satisfaction score was higher in the TM group. At the end of the study, 67.9% of the overall participants agreed to use TM as a mode of future FU. Although the mean indirect costs of illness were numerically higher in the SF group, the out-of-pocket costs for health care services other than those provided by the public sector were similar between the two groups. The total number of FU was similar in the 2 groups. However, significantly more patients in the TM group requested change mode of FU. The proportion of patients requiring hospitalization was also higher in the TM group. Not being in LLDAS at baseline was the independent predictor of hospitalization (OR 3.4, 95%CI 1.20-9.65). None of the participants has been tested positive for COVID-19 throughout the study.
Conclusion: The results of the study suggest that TM delivered care could help maintaining disease control and psychosocial wellbeing during the pandemic, but it needs to be complemented by physical visits as required, particularly in those with unstable disease.
.jpg)


Disclosures: H. SO, None; E. Chow, None; I. Cheng, None; X. Lau, None; T. Li, None; C. Szeto, None; L. Tam, AbbVie, Amgen, Boehringer-Ingelheim, Eli Lilly, GlaxoSmithKlein(GSK), Janssen, Novartis, Pfizer, Sanofi, AstraZeneca.

