Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0650: The Association of Urinary C9 to CD59 Ratio with Tubulointerstitial Fibrosis in Lupus Nephritis
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- SW
Shudan Wang, MD, MS
Albert Einstein College of Medicine
New York, NY, United States
Abstract Poster Presenter(s)
Shudan Wang1, Masako Suzuki2, Daming Shao3, Muhammad Hamza Bukhari4, Brianna Lally5, Michael Belmont6, Jennifer Aguilan2, Simone Sidoli2, Anna Broder7 and J. Michelle Kahlenberg8, 1Montefiore Medical Center / Albert Einstein College of Medicine, New York, NY, 2Albert Einstein College of Medicine, Bronx, NY, 3Albert Einstein College of Medicine/Jacobi Medical Center, Bronx, NY, 4Norwalk Hospital/Yale University, Norkwalk, VA, 5University of Wisconsin Hospitals and Clinics, Madison, WI, 6NYU Grossman School of Medicine, New York, NY, 7Hackensack University Medical Center, Hackensack, NJ, 8University of Michigan, Ann Arbor, MI
Background/Purpose: Animal and human studies suggest that terminal complement activation has a pathogenic role in tubulointerstitial fibrosis, which is a strong predictor of irreversible kidney damage in lupus nephritis (LN). Our pilot study linked membrane attack complex (MAC) deposition in the tubules with interstitial fibrosis/tubular atrophy (IFTA). The assembly of MAC by Complement C5b to C9 on the cell membrane leads to cytotoxic pores and cell lysis while CD59 inhibits MAC formation by preventing C9 from joining the complex. It has been shown that the imbalance between complement activation and inhibition predisposes lupus patients to disease manifestations such as preeclampsia but the interplay between complement activators and regulators in LN has not been studied. We hypothesize that an imbalance between uncontrolled MAC activation and CD59 inhibition is associated with IFTA in LN. As urinary complement biomarkers offer a non-invasive method of evaluating kidney pathology, we compared urinary C9-to-CD59 ratio between LN patients with moderate/severe IFTA versus none/mild IFTA.
Methods: Urine samples from 46 adults and pediatric lupus patients with clinically indicated kidney biopsy performed between 2010 and 2019 were included. Proteomics analysis was performed using mass spectrometry (Orbitrap Fusion Lumos, Thermo Scientific) and processed by the Proteome Discoverer. The urinary proteins detected were normalized by urine creatinine excretion. IFTA was categorized as none/mild (< 25% of interstitium affected) versus moderate/severe (≥ 25% of interstitium affected). Log-transformed urinary C9, CD59 and C9-to-CD59 ratio were compared between the two groups.
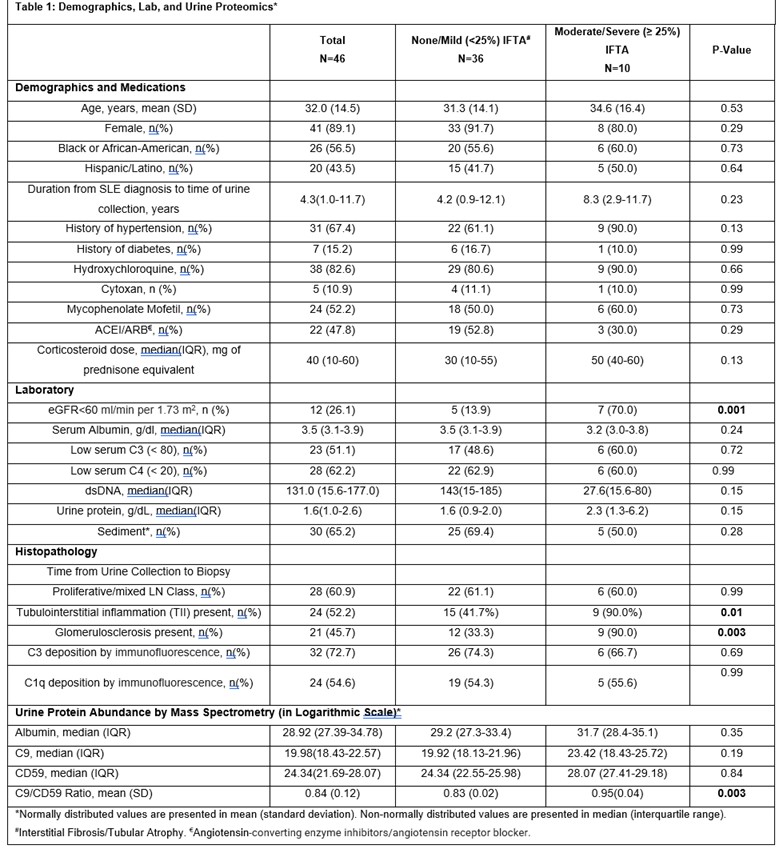
Results: Of the 46 LN patients included in the study, 41 (89.1%) were women, 20 (43.5%) self-identified as Hispanic or Latino, and 26 (56.5%) self-identified as Black or African American. Ten of the 46 (21.7%) LN patients had moderate/severe IFTA on kidney biopsy (Table 1). Moderate/severe IFTA group included a higher proportion of patients with reduced eGFR < 60 ml/min per 1.73 m2 [70.0% vs. 13.9%, p=0.001], a higher proportion of patients with tubulointerstitial inflammation [90.0% vs. 41.7%, p= 0.01] and a higher proportion of patients with glomerulosclerosis [90.0% vs. 33.3%, p=0.003]. There was no difference with respect to age, race, sex, medication use, serum C3 and C4, urine protein, and LN class between the two groups.
Proteomic analysis showed increased urinary C9-to-CD59 ratio in the moderate/severe IFTA group vs none/mild IFTA group [mean (standard deviation) 0.95(0.04) vs 0.83(0.02), p=0.003], Figure 1. There was no difference in urinary C9 and CD59 abundance between the groups (Figure 2, Table 1). Urinary C9-to-CD59 ratio was not associated with albuminuria (rho=-0.05, p= 0.75), suggesting increased urinary complement products are not likely a result of a compromised kidney barrier alone.
Conclusion: We observed a higher urinary C9-to-CD59 ratio in LN patients with fibrosis suggesting that terminal complement activation is amplified in relation to MAC inhibition. The imbalance between complement activation and inhibition may play a role in tubulointerstitial fibrosis.
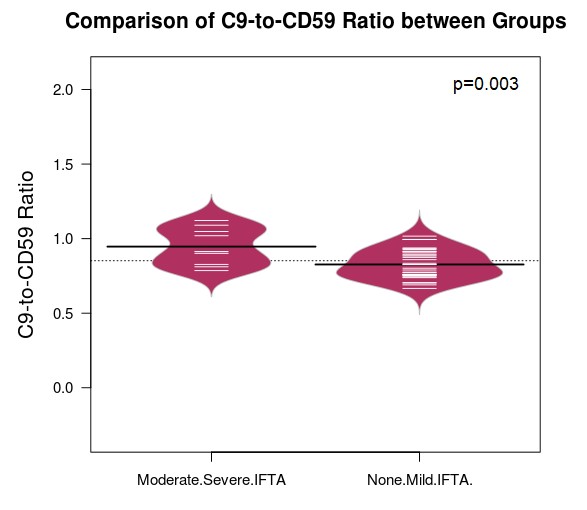 Black line indicates the mean of each group. White line represents each subject.
Black line indicates the mean of each group. White line represents each subject.
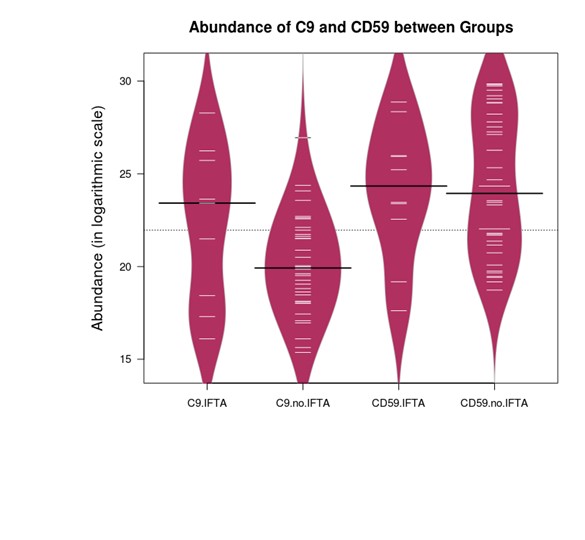 Black line indicates the median of each group. White line represents each subject.
Black line indicates the median of each group. White line represents each subject.
 References:
References:
1. Wang S, Wu M, Chiriboga L, et al. Membrane attack complex (MAC) deposition in renal tubules is associated with interstitial fibrosis and tubular atrophy: a pilot study. Lupus Sci Med. 2022 Jan;9(1).
2. Salmon J, Heuser C, Triebwasser M et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011 Mar;8(3)
3. Hsu, SIH, & Couser, W. G. (2003). Chronic progression of tubulointerstitial damage in proteinuric renal disease is mediated by complement activation: A therapeutic role for complement inhibitors. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S186-91.
Disclosures: S. Wang, None; M. Suzuki, None; D. Shao, None; M. Bukhari, None; B. Lally, None; M. Belmont, None; J. Aguilan, None; S. Sidoli, None; A. Broder, None; J. Kahlenberg, Q32 Bio, Celgene/Bristol Myers Squibb, Ventus Therapeutics, Rome Therapeutics, Janssen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Boehringer Ingelheim.
Background/Purpose: Animal and human studies suggest that terminal complement activation has a pathogenic role in tubulointerstitial fibrosis, which is a strong predictor of irreversible kidney damage in lupus nephritis (LN). Our pilot study linked membrane attack complex (MAC) deposition in the tubules with interstitial fibrosis/tubular atrophy (IFTA). The assembly of MAC by Complement C5b to C9 on the cell membrane leads to cytotoxic pores and cell lysis while CD59 inhibits MAC formation by preventing C9 from joining the complex. It has been shown that the imbalance between complement activation and inhibition predisposes lupus patients to disease manifestations such as preeclampsia but the interplay between complement activators and regulators in LN has not been studied. We hypothesize that an imbalance between uncontrolled MAC activation and CD59 inhibition is associated with IFTA in LN. As urinary complement biomarkers offer a non-invasive method of evaluating kidney pathology, we compared urinary C9-to-CD59 ratio between LN patients with moderate/severe IFTA versus none/mild IFTA.
Methods: Urine samples from 46 adults and pediatric lupus patients with clinically indicated kidney biopsy performed between 2010 and 2019 were included. Proteomics analysis was performed using mass spectrometry (Orbitrap Fusion Lumos, Thermo Scientific) and processed by the Proteome Discoverer. The urinary proteins detected were normalized by urine creatinine excretion. IFTA was categorized as none/mild (< 25% of interstitium affected) versus moderate/severe (≥ 25% of interstitium affected). Log-transformed urinary C9, CD59 and C9-to-CD59 ratio were compared between the two groups.
Results: Of the 46 LN patients included in the study, 41 (89.1%) were women, 20 (43.5%) self-identified as Hispanic or Latino, and 26 (56.5%) self-identified as Black or African American. Ten of the 46 (21.7%) LN patients had moderate/severe IFTA on kidney biopsy (Table 1). Moderate/severe IFTA group included a higher proportion of patients with reduced eGFR < 60 ml/min per 1.73 m2 [70.0% vs. 13.9%, p=0.001], a higher proportion of patients with tubulointerstitial inflammation [90.0% vs. 41.7%, p= 0.01] and a higher proportion of patients with glomerulosclerosis [90.0% vs. 33.3%, p=0.003]. There was no difference with respect to age, race, sex, medication use, serum C3 and C4, urine protein, and LN class between the two groups.
Proteomic analysis showed increased urinary C9-to-CD59 ratio in the moderate/severe IFTA group vs none/mild IFTA group [mean (standard deviation) 0.95(0.04) vs 0.83(0.02), p=0.003], Figure 1. There was no difference in urinary C9 and CD59 abundance between the groups (Figure 2, Table 1). Urinary C9-to-CD59 ratio was not associated with albuminuria (rho=-0.05, p= 0.75), suggesting increased urinary complement products are not likely a result of a compromised kidney barrier alone.
Conclusion: We observed a higher urinary C9-to-CD59 ratio in LN patients with fibrosis suggesting that terminal complement activation is amplified in relation to MAC inhibition. The imbalance between complement activation and inhibition may play a role in tubulointerstitial fibrosis.
 Black line indicates the mean of each group. White line represents each subject.
Black line indicates the mean of each group. White line represents each subject.  Black line indicates the median of each group. White line represents each subject.
Black line indicates the median of each group. White line represents each subject.  References:
References:1. Wang S, Wu M, Chiriboga L, et al. Membrane attack complex (MAC) deposition in renal tubules is associated with interstitial fibrosis and tubular atrophy: a pilot study. Lupus Sci Med. 2022 Jan;9(1).
2. Salmon J, Heuser C, Triebwasser M et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011 Mar;8(3)
3. Hsu, SIH, & Couser, W. G. (2003). Chronic progression of tubulointerstitial damage in proteinuric renal disease is mediated by complement activation: A therapeutic role for complement inhibitors. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S186-91.
Disclosures: S. Wang, None; M. Suzuki, None; D. Shao, None; M. Bukhari, None; B. Lally, None; M. Belmont, None; J. Aguilan, None; S. Sidoli, None; A. Broder, None; J. Kahlenberg, Q32 Bio, Celgene/Bristol Myers Squibb, Ventus Therapeutics, Rome Therapeutics, Janssen, AstraZeneca, Eli Lilly, GlaxoSmithKline, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Boehringer Ingelheim.

