Back
Poster Session B
Reproductive health
Session: (0939–0969) Reproductive Issues in Rheumatic Disorders Poster
0961: The Use of Belimumab Before and During Pregnancy in Patients with Systemic Lupus Erythematosus: An Italian Multicenter Case-series
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Francesca Crisafulli, MD
ASST Spedali Civili and University of Brescia
Brescia, Brescia, Italy
Abstract Poster Presenter(s)
Francesca Crisafulli1, MARIA CHIARA GERARDI2, Maria Letizia Urban3, Margherita Zen4, Melissa Padovan5, Valentina Canti6, Emanuela Praino7, Cecilia Nalli1, Francesca Ruffilli5, Francesca Saccon8, Micaela Fredi9, Liala Moschetti1, Giacomo Emmi3, Luca Iaccarino4, Andrea Doria10, Leonardo Santo7, Franco Franceschini1, Laura Andreoli1 and Angela Tincani11, 1Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia, Brescia, Italy, 2Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia and Rheumatology Unit, ASST G.O.M. Niguarda, Milan, Milano, Italy, 3Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy, 4Unit of Rheumatology, Department of Medicine, University of Padova, Padova, Italy, 5Rheumatology Unit, Azienda Ospedaliero-Universitaria of Ferrara, Ferrara, Italy, 6Division of Immunology, IRCCS San Raffaele Institute, Milano, Italy, 7Rheumatology Unit, Presidio Ospedaliero ‘Mons. Dimiccoli’, Barletta, Italy, 8Unit of Rheumatology, Department of Medicine, University of Padova and Fondazione Villa Salus, Mestra, Venezia, Italy, 9Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia, Manerbio, Italy, 10Rheumatology Unit, Department of Medicine (DIMED), University of Padova, Via Giustiniani, 2, 35128 Padova, Italy., Padova, Italy, 11Rheumatology and Clinical Immunology Unit, ASST Spedali Civili and University of Brescia, Gussago, Italy
Background/Purpose: Belimumab (BEL) is an anti-BLyS monoclonal antibody approved for SLE treatment. As few data about BEL use with regard to pregnancy are available, the aim of this study is to describe pregnancies exposed to BEL either preconceptionally or during pregnancy.
Methods: Data of prospectively-followed pregnancies (2014-2021) in SLE patients treated with BEL in 6 Italian centers where retrospectively collected, focusing on disease activity and outcome. Continuous data were reported as median (interquartile range).
Results: Twenty-one pregnancies in 21 SLE patients were collected (median age at conception: 34 [31-38] years; 13 in primigravidae (62%); 16 planned (76%); 18 spontaneous (86%)).
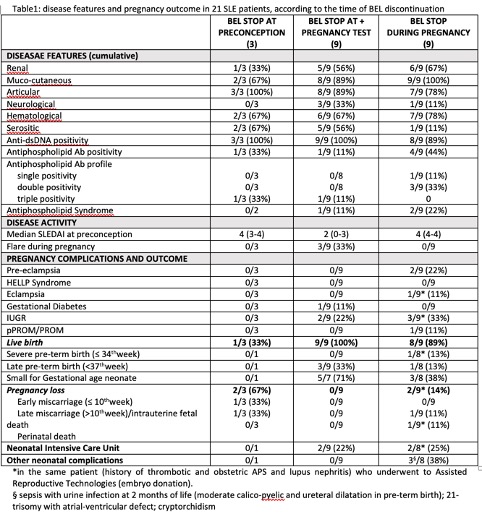
BEL (14 intravenous, 7 subcutaneous) was stopped in 3 cases preconceptionally, in 9 at positive pregnancy test and in 9 during pregnancy (4 during the 1st trimester, 3 during the 2nd trimester and 2 during the 3rd trimester) (Table 1). The use of BEL during pregnancy had been agreed with the patient during preconception counselling.
Other treatments during pregnancy were: prednisone (90%); antimalarials (81%); azathioprine (48%); calcineurin-inhibitors (29%); low-dose acetylsalicylic acid (86%); low molecular weight heparin (57%).
At preconception visit, the median SLEDAI was 4 [2-4].
Three pregnancies had a flare (1 during the 2nd and the 3rd trimester; 2 during the 3rd trimester), all in patients who withdrew BEL at positive pregnancy test.
Live-births were 86%.
Two miscarriages (at 7th and 11th week) and 1 intrauterine fetal death (at 37th week; 21-trisomy with atrio-ventricular defect) occurred. One perinatal death occurred (in a patient with thrombotic+obstetric APS and lupus nephritis who underwent heterologous assisted reproductive technology -embryodonation- and developed eclampsia with cerebral haemorrage at 25thweek; an urgent cesarean section was performed and the newborn died after 3 days).
Two pre-eclampsia were registered: 1 at 38th week in a patient with history of lupus nephritis, double aPL positivity and active disease and 1 at 33th week in a patient with cardiovascular risk factors (hypertension, obesity, cigarette smoke) and a history of pre-eclampsia in a previous pregnancy.
Four newborns were hospitalized in Intensive Care Unit (1 milk protein intolerance; 1 desaturation; 2 prematurity). One urine infection with sepsis occurred at 2 months in a baby with calico-pyelic and ureteral dilatation at birth (pre-term birth).
Eleven newborns received vaccinations according to national schedule (7 missing data).
Conclusion: In this case series, the use of BEL during pregnancy was discussed with some patients with a severe disease phenotype and at high risk of having a flare during pregnancy, in order to favor disease remission. While more data are needed, the timing of discontinuation should be individualized according to the single patient's characteristics and preferences, with a risk-benefit evaluation.
Disclosures: F. Crisafulli, UCB, Novartis, Eli Lilly; M. GERARDI, None; M. Urban, None; M. Zen, GlaxoSmithKlein(GSK); M. Padovan, None; V. Canti, None; E. Praino, None; C. Nalli, None; F. Ruffilli, None; F. Saccon, None; M. Fredi, GlaxoSmithKlein(GSK); L. Moschetti, None; G. Emmi, GlaxoSmithKlein(GSK), Sobi, Novartis; L. Iaccarino, GSK; A. Doria, GlaxoSmithKlein(GSK); L. Santo, None; F. Franceschini, None; L. Andreoli, GlaxoSmithKlein(GSK), Janssen, Novartis, UCB, Werfen; A. Tincani, GlaxoSmithKlein(GSK), UCB.
Background/Purpose: Belimumab (BEL) is an anti-BLyS monoclonal antibody approved for SLE treatment. As few data about BEL use with regard to pregnancy are available, the aim of this study is to describe pregnancies exposed to BEL either preconceptionally or during pregnancy.
Methods: Data of prospectively-followed pregnancies (2014-2021) in SLE patients treated with BEL in 6 Italian centers where retrospectively collected, focusing on disease activity and outcome. Continuous data were reported as median (interquartile range).
Results: Twenty-one pregnancies in 21 SLE patients were collected (median age at conception: 34 [31-38] years; 13 in primigravidae (62%); 16 planned (76%); 18 spontaneous (86%)).
BEL (14 intravenous, 7 subcutaneous) was stopped in 3 cases preconceptionally, in 9 at positive pregnancy test and in 9 during pregnancy (4 during the 1st trimester, 3 during the 2nd trimester and 2 during the 3rd trimester) (Table 1). The use of BEL during pregnancy had been agreed with the patient during preconception counselling.
Other treatments during pregnancy were: prednisone (90%); antimalarials (81%); azathioprine (48%); calcineurin-inhibitors (29%); low-dose acetylsalicylic acid (86%); low molecular weight heparin (57%).
At preconception visit, the median SLEDAI was 4 [2-4].
Three pregnancies had a flare (1 during the 2nd and the 3rd trimester; 2 during the 3rd trimester), all in patients who withdrew BEL at positive pregnancy test.
Live-births were 86%.
Two miscarriages (at 7th and 11th week) and 1 intrauterine fetal death (at 37th week; 21-trisomy with atrio-ventricular defect) occurred. One perinatal death occurred (in a patient with thrombotic+obstetric APS and lupus nephritis who underwent heterologous assisted reproductive technology -embryodonation- and developed eclampsia with cerebral haemorrage at 25thweek; an urgent cesarean section was performed and the newborn died after 3 days).
Two pre-eclampsia were registered: 1 at 38th week in a patient with history of lupus nephritis, double aPL positivity and active disease and 1 at 33th week in a patient with cardiovascular risk factors (hypertension, obesity, cigarette smoke) and a history of pre-eclampsia in a previous pregnancy.
Four newborns were hospitalized in Intensive Care Unit (1 milk protein intolerance; 1 desaturation; 2 prematurity). One urine infection with sepsis occurred at 2 months in a baby with calico-pyelic and ureteral dilatation at birth (pre-term birth).
Eleven newborns received vaccinations according to national schedule (7 missing data).
Conclusion: In this case series, the use of BEL during pregnancy was discussed with some patients with a severe disease phenotype and at high risk of having a flare during pregnancy, in order to favor disease remission. While more data are needed, the timing of discontinuation should be individualized according to the single patient's characteristics and preferences, with a risk-benefit evaluation.

Disclosures: F. Crisafulli, UCB, Novartis, Eli Lilly; M. GERARDI, None; M. Urban, None; M. Zen, GlaxoSmithKlein(GSK); M. Padovan, None; V. Canti, None; E. Praino, None; C. Nalli, None; F. Ruffilli, None; F. Saccon, None; M. Fredi, GlaxoSmithKlein(GSK); L. Moschetti, None; G. Emmi, GlaxoSmithKlein(GSK), Sobi, Novartis; L. Iaccarino, GSK; A. Doria, GlaxoSmithKlein(GSK); L. Santo, None; F. Franceschini, None; L. Andreoli, GlaxoSmithKlein(GSK), Janssen, Novartis, UCB, Werfen; A. Tincani, GlaxoSmithKlein(GSK), UCB.

