Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0647: Transcriptomic Analysis of Lupus Nephritis Kidneys Identifies Molecular Endotypes
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- KK
Kathryn Kingsmore Allison, PhD
AMPEL BioSolutions
Charlottesville, VA, United States
Abstract Poster Presenter(s)
Kathryn Kingsmore Allison1, Sneha Shrotri1, Prathyusha Bachali2, Nan Shen3, Amrie Grammer4 and Peter Lipsky1, 1AMPEL BioSolutions, Charlottesville, VA, 2AMPEL BioSolutions, Redmond, WA, 3Shanghai Jiang Tong University School of Medicine, Shanghai, China, 4AMPEL LLC, Charlottesville, VA
Background/Purpose: Predicting the course or response to treatment of lupus nephritis (LN) from standard renal biopsies is problematic. We, therefore sought to understand the molecular endotypes of LN by analyzing bulk RNA from renal biopsies.
Methods: Gene expression of biopsies from 76 kidneys derived from patients with LN was analyzed for enrichment of informative modules of co-expressed genes using Gene Set Variation Analysis (GSVA) and grouped into four clusters with hierarchical clustering. Gene modules identifying immune/inflammatory cells as well as resident kidney cells and metabolic activities were employed. Kidney biopsy histology and immunofluorescence were scored by a blinded clinical pathologist.
Results: Analysis of LN gene expression with GSVA revealed four distinct endotypes of LN (Fig. 1a), which could be ordered from least to most severe based on the profile of abnormal features (Fig. 1b). The least severe cluster (coral) exhibited minimal immune cell infiltrates or changes to kidney cell or metabolic signature expression. The second cluster (yellow) had increased expression of immune/inflammatory cell signatures, with minimal changes to kidney cell or metabolic signatures. The third cluster (purple) exhibited both increased expression of immune cell signatures and decreased expression of kidney cell and metabolic signatures. The final cluster (black) exhibited minimal immune cell gene expression, but decreased metabolism signatures and increased endothelial cell, fibroblast, and mesangial cell signatures. Of the LN samples with paired histology, 24/33 samples with proliferative LN were found in clusters with increased immune/inflammatory cell signatures (15/33 in the purple cluster, 9/33 in the yellow cluster) (Fig. 2a). Notably, the percentage of patients with IgA deposition on the glomerular basement membrane was highest in the purple cluster, and significantly lower in the coral cluster (Fig. 2b). Moreover, the percentage of patients with active disease determined by SLEDAI was lowest in the coral cluster (Fig. 2c). Mean renal activity and chronicity indices were not significantly different between clusters (Fig. 2d-e).
Conclusion: Transcriptomic analyses suggest distinct endotypes of LN, including: 1) minimal disease; 2) inflammatory disease without kidney cell damage or metabolic dysfunction; 3) inflammatory disease with kidney cell and metabolic dysfunction; and 4) markedly decreased kidney cell and metabolic function with little inflammation. Even though there is modest association with histologic phenotypes, the molecular endotypes suggest progression of LN from acute inflammatory to chronic dysfunctional kidney disease. The molecular endotypes of LN may be an important way to stage LN and provide useful information to guide therapy.
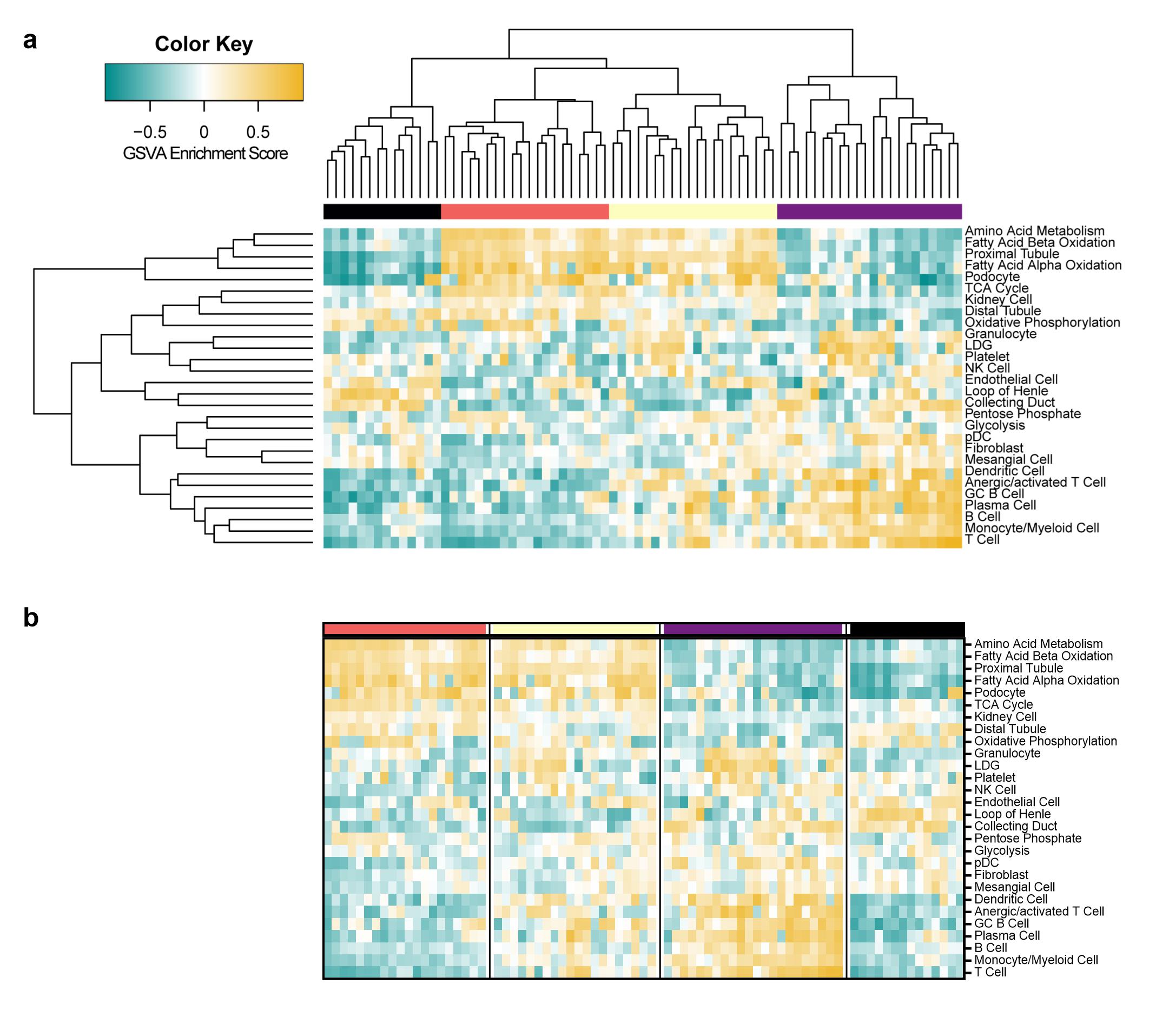 Figure 1 | Clustering of GSVA enrichment scores in lupus kidneys reveals four distinct endotypes of patients with LN. (a) Row and column hierarchical clustering of 76 patients with LN into four groups based upon gene expression of cellular and pathway gene modules. (b) Reordered clustering of LN patients in order of molecular disease severity from least to greatest. The columns represent individual patients that are grouped into four clusters (black, coral, yellow, and purple). The rows represent gene modules indicative of immune/inflammatory cells, non-hematopoietic cells, and cellular metabolism.
Figure 1 | Clustering of GSVA enrichment scores in lupus kidneys reveals four distinct endotypes of patients with LN. (a) Row and column hierarchical clustering of 76 patients with LN into four groups based upon gene expression of cellular and pathway gene modules. (b) Reordered clustering of LN patients in order of molecular disease severity from least to greatest. The columns represent individual patients that are grouped into four clusters (black, coral, yellow, and purple). The rows represent gene modules indicative of immune/inflammatory cells, non-hematopoietic cells, and cellular metabolism.
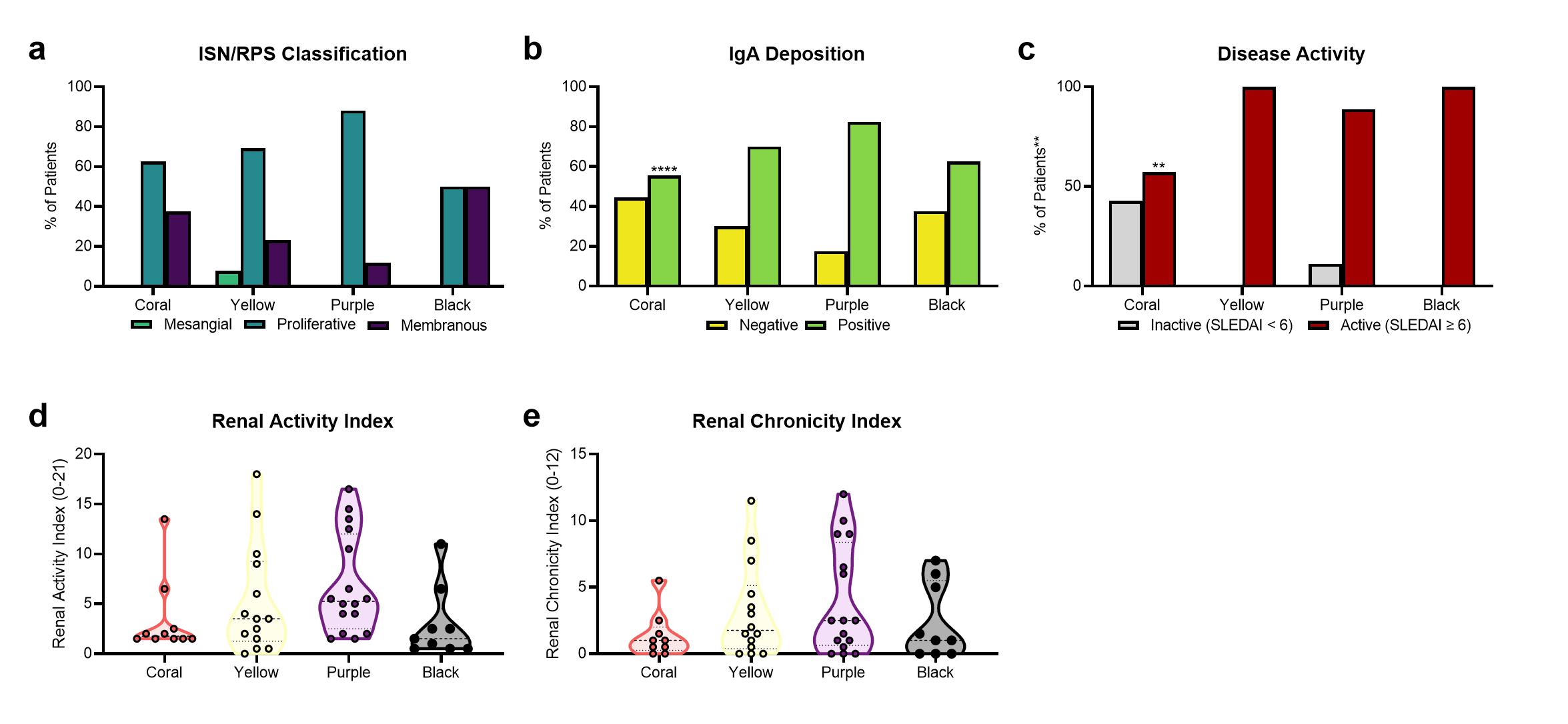 Figure 2 | Comparison of molecular endotypes with clinical features reveals some correlation between gene expression and histology. Distribution of (a) ISN/RPS classes in 46 patients with LN, (b) positive or negative IgA deposition in 44 patients with LN, (c) inactive or active SLEDAI in 32 patients with LN, (d) renal activity index in 49 patients with LN, and (e) renal chronicity index in 48 patients with LN among the LN endotypes. In (a-c) significant differences in expected and observed frequencies between coral, the “least abnormal” LN endotype, and all other clusters (denoted with asterisk above bars) for (a) proliferative LN, (b) positive IgA deposition, and (c) active SLEDAI were identified by Chi Square Test. The likelihood of having proliferative LN in the coral cluster was not significantly different than the other clusters. The likelihood (odds ratio) of having positive IgA deposition in the coral cluster is 0.43 (p < 0.0001) as compared to the other three clusters. The likelihood (odds ratio) of having active SLE (SLEDAI ≥ 6) in the coral cluster is 0.06 (p < 0.01) as compared to the other three clusters. In (a-c) significant associations between the categorical variables and all clusters (denoted with asterisks on the y-axis) were identified using Chi Square Test of Independence. In (d-e) Significant differences in mean of the renal activity or renal chronicity index between the coral cluster and each other cluster was assessed by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons. **, p < 0.01, ****, p < 0.0001.
Figure 2 | Comparison of molecular endotypes with clinical features reveals some correlation between gene expression and histology. Distribution of (a) ISN/RPS classes in 46 patients with LN, (b) positive or negative IgA deposition in 44 patients with LN, (c) inactive or active SLEDAI in 32 patients with LN, (d) renal activity index in 49 patients with LN, and (e) renal chronicity index in 48 patients with LN among the LN endotypes. In (a-c) significant differences in expected and observed frequencies between coral, the “least abnormal” LN endotype, and all other clusters (denoted with asterisk above bars) for (a) proliferative LN, (b) positive IgA deposition, and (c) active SLEDAI were identified by Chi Square Test. The likelihood of having proliferative LN in the coral cluster was not significantly different than the other clusters. The likelihood (odds ratio) of having positive IgA deposition in the coral cluster is 0.43 (p < 0.0001) as compared to the other three clusters. The likelihood (odds ratio) of having active SLE (SLEDAI ≥ 6) in the coral cluster is 0.06 (p < 0.01) as compared to the other three clusters. In (a-c) significant associations between the categorical variables and all clusters (denoted with asterisks on the y-axis) were identified using Chi Square Test of Independence. In (d-e) Significant differences in mean of the renal activity or renal chronicity index between the coral cluster and each other cluster was assessed by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons. **, p < 0.01, ****, p < 0.0001.
Disclosures: K. Kingsmore Allison, None; S. Shrotri, None; P. Bachali, None; N. Shen, None; A. Grammer, None; P. Lipsky, None.
Background/Purpose: Predicting the course or response to treatment of lupus nephritis (LN) from standard renal biopsies is problematic. We, therefore sought to understand the molecular endotypes of LN by analyzing bulk RNA from renal biopsies.
Methods: Gene expression of biopsies from 76 kidneys derived from patients with LN was analyzed for enrichment of informative modules of co-expressed genes using Gene Set Variation Analysis (GSVA) and grouped into four clusters with hierarchical clustering. Gene modules identifying immune/inflammatory cells as well as resident kidney cells and metabolic activities were employed. Kidney biopsy histology and immunofluorescence were scored by a blinded clinical pathologist.
Results: Analysis of LN gene expression with GSVA revealed four distinct endotypes of LN (Fig. 1a), which could be ordered from least to most severe based on the profile of abnormal features (Fig. 1b). The least severe cluster (coral) exhibited minimal immune cell infiltrates or changes to kidney cell or metabolic signature expression. The second cluster (yellow) had increased expression of immune/inflammatory cell signatures, with minimal changes to kidney cell or metabolic signatures. The third cluster (purple) exhibited both increased expression of immune cell signatures and decreased expression of kidney cell and metabolic signatures. The final cluster (black) exhibited minimal immune cell gene expression, but decreased metabolism signatures and increased endothelial cell, fibroblast, and mesangial cell signatures. Of the LN samples with paired histology, 24/33 samples with proliferative LN were found in clusters with increased immune/inflammatory cell signatures (15/33 in the purple cluster, 9/33 in the yellow cluster) (Fig. 2a). Notably, the percentage of patients with IgA deposition on the glomerular basement membrane was highest in the purple cluster, and significantly lower in the coral cluster (Fig. 2b). Moreover, the percentage of patients with active disease determined by SLEDAI was lowest in the coral cluster (Fig. 2c). Mean renal activity and chronicity indices were not significantly different between clusters (Fig. 2d-e).
Conclusion: Transcriptomic analyses suggest distinct endotypes of LN, including: 1) minimal disease; 2) inflammatory disease without kidney cell damage or metabolic dysfunction; 3) inflammatory disease with kidney cell and metabolic dysfunction; and 4) markedly decreased kidney cell and metabolic function with little inflammation. Even though there is modest association with histologic phenotypes, the molecular endotypes suggest progression of LN from acute inflammatory to chronic dysfunctional kidney disease. The molecular endotypes of LN may be an important way to stage LN and provide useful information to guide therapy.
 Figure 1 | Clustering of GSVA enrichment scores in lupus kidneys reveals four distinct endotypes of patients with LN. (a) Row and column hierarchical clustering of 76 patients with LN into four groups based upon gene expression of cellular and pathway gene modules. (b) Reordered clustering of LN patients in order of molecular disease severity from least to greatest. The columns represent individual patients that are grouped into four clusters (black, coral, yellow, and purple). The rows represent gene modules indicative of immune/inflammatory cells, non-hematopoietic cells, and cellular metabolism.
Figure 1 | Clustering of GSVA enrichment scores in lupus kidneys reveals four distinct endotypes of patients with LN. (a) Row and column hierarchical clustering of 76 patients with LN into four groups based upon gene expression of cellular and pathway gene modules. (b) Reordered clustering of LN patients in order of molecular disease severity from least to greatest. The columns represent individual patients that are grouped into four clusters (black, coral, yellow, and purple). The rows represent gene modules indicative of immune/inflammatory cells, non-hematopoietic cells, and cellular metabolism. Figure 2 | Comparison of molecular endotypes with clinical features reveals some correlation between gene expression and histology. Distribution of (a) ISN/RPS classes in 46 patients with LN, (b) positive or negative IgA deposition in 44 patients with LN, (c) inactive or active SLEDAI in 32 patients with LN, (d) renal activity index in 49 patients with LN, and (e) renal chronicity index in 48 patients with LN among the LN endotypes. In (a-c) significant differences in expected and observed frequencies between coral, the “least abnormal” LN endotype, and all other clusters (denoted with asterisk above bars) for (a) proliferative LN, (b) positive IgA deposition, and (c) active SLEDAI were identified by Chi Square Test. The likelihood of having proliferative LN in the coral cluster was not significantly different than the other clusters. The likelihood (odds ratio) of having positive IgA deposition in the coral cluster is 0.43 (p < 0.0001) as compared to the other three clusters. The likelihood (odds ratio) of having active SLE (SLEDAI ≥ 6) in the coral cluster is 0.06 (p < 0.01) as compared to the other three clusters. In (a-c) significant associations between the categorical variables and all clusters (denoted with asterisks on the y-axis) were identified using Chi Square Test of Independence. In (d-e) Significant differences in mean of the renal activity or renal chronicity index between the coral cluster and each other cluster was assessed by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons. **, p < 0.01, ****, p < 0.0001.
Figure 2 | Comparison of molecular endotypes with clinical features reveals some correlation between gene expression and histology. Distribution of (a) ISN/RPS classes in 46 patients with LN, (b) positive or negative IgA deposition in 44 patients with LN, (c) inactive or active SLEDAI in 32 patients with LN, (d) renal activity index in 49 patients with LN, and (e) renal chronicity index in 48 patients with LN among the LN endotypes. In (a-c) significant differences in expected and observed frequencies between coral, the “least abnormal” LN endotype, and all other clusters (denoted with asterisk above bars) for (a) proliferative LN, (b) positive IgA deposition, and (c) active SLEDAI were identified by Chi Square Test. The likelihood of having proliferative LN in the coral cluster was not significantly different than the other clusters. The likelihood (odds ratio) of having positive IgA deposition in the coral cluster is 0.43 (p < 0.0001) as compared to the other three clusters. The likelihood (odds ratio) of having active SLE (SLEDAI ≥ 6) in the coral cluster is 0.06 (p < 0.01) as compared to the other three clusters. In (a-c) significant associations between the categorical variables and all clusters (denoted with asterisks on the y-axis) were identified using Chi Square Test of Independence. In (d-e) Significant differences in mean of the renal activity or renal chronicity index between the coral cluster and each other cluster was assessed by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons. **, p < 0.01, ****, p < 0.0001.Disclosures: K. Kingsmore Allison, None; S. Shrotri, None; P. Bachali, None; N. Shen, None; A. Grammer, None; P. Lipsky, None.

