Back
Poster Session B
Vasculitis
Session: (1071–1093) Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
1071: Trimethoprim Sulfamethoxazole Use in Patients with Granulomatosis with Polyangiitis Treated with Rituximab
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- AM
Arielle Mendel, MD, FRCPC, MSc
McGill University Health Centre
Montréal, QC, Canada
Abstract Poster Presenter(s)
Arielle Mendel1, Hassan Behlouli2, Cristiano Moura2, Evelyne Vinet1, Jeffrey Curtis3 and Sasha Bernatsky2, 1McGill University Health Centre, Montréal, QC, Canada, 2Research Institute of the McGill University Health Centre, Montréal, QC, Canada, 3Division of Clinical Immunology & Rheumatology, University of Alabama at Birmingham, Birmingham, AL
Background/Purpose: Trimethoprim sulfamethoxazole (TMP-SMX) prophylaxis is recommended during induction of ANCA-associated vasculitis (AAV) to prevent pneumocystis jirovecii pneumonia (PJP), and may also reduce the risk of other serious infections. Our objective was to describe the frequency and persistence of TMP-SMX prophylaxis in patients with granulomatosis with polyangiitis (GPA) treated with rituximab (RTX), and determine factors associated with TMP-SMX use.
Methods: We identified adults meeting prior ICD case definitions for GPA from the MarketScan United States Commercial Claims and Medicaid health care databases (2011-2019). Those with a RTX claim coincident with or following the first GPA diagnostic code were included. The primary outcome was baseline TMP-SMX prophylaxis, defined as a minimum 28-day TMP-SMX prescription, overlapping with the first RTX treatment or dispensed within the following month. Covariates included age, sex, calendar year, glucocorticoid (GC) use in the 30 days prior to RTX, cyclophosphamide use, hospital admission in the 6 months prior to RTX, and baseline conditions (sinusitis, lung disease, chronic kidney disease, dialysis). We estimated the frequency and persistence of TMP-SMX use over time, allowing a prescription refill gap of 14 days. Univariable and multivariable logistic regressions were performed to identify factors associated with baseline TMP-SMX. We tested for calendar-year trends in TMP-SMX use.
Results: We studied 1921 RTX-treated GPA patients, including 222 (12%) Medicaid beneficiaries (Table 1). At the time of RTX initiation, mean age was 50 (SD 16) years, 54% were female, and median time from the first GPA diagnostic code was 67 days (IQR 16, 290). TMP-SMX was dispensed to 440 (23%) subjects at baseline. An additional 175 (9%) started TMP-SMX later in the 6-month period following RTX. TMP-SMX use increased over time, from 19% in 2011 to 28% in 2019 (p trend=0.023). Prophylaxis persisted for a median of 130 days (IQR 85, 205), with 126/440 (31%) TMP-SMX users persisting for at least 6 months. In multivariable logistic regression analyses, female sex (OR 0.68, 95% CI 0.50-0.80) was negatively associated with baseline prophylaxis, while prior hospitalization (OR 1.46, 95% CI 1.1-1.9) and GC use (OR 3.42, 95% CI 2.7-4.4) were positively associated with prophylaxis (Table 2).
Conclusion: TMP-SMX prophylaxis is dispensed to less than a quarter of GPA patients at the time of RTX, but has increased over calendar time. Recent GC had the greatest association with prophylaxis. Further work is needed to confirm if TMP-SMX reduces not only the incidence of PJP but also all-cause serious infections in patients with GPA.
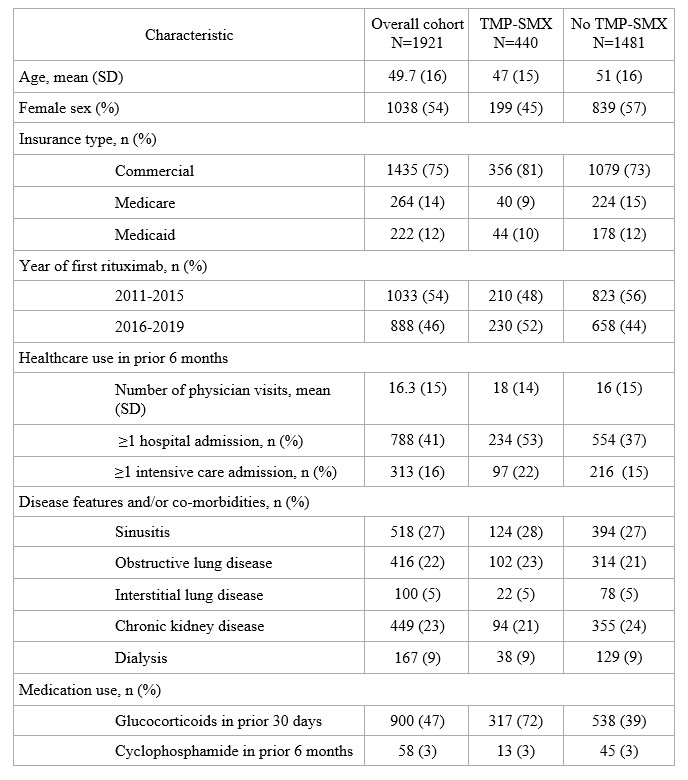 Table 1. Characteristics of GPA cohort at the time of rituximab, overall and according to baseline trimethoprim sulfamethoxazole (TMP-SMX) use
Table 1. Characteristics of GPA cohort at the time of rituximab, overall and according to baseline trimethoprim sulfamethoxazole (TMP-SMX) use
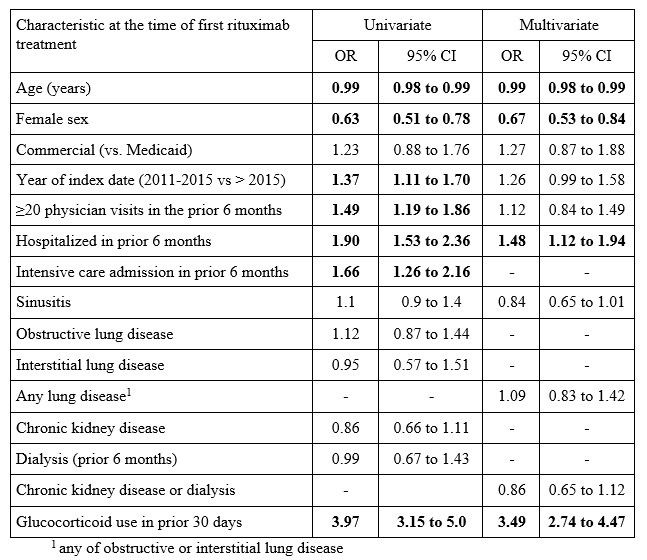 Table 2. Univariate and multivariate logistic regression analysis of factors associated with baseline TMP-SMX use
Table 2. Univariate and multivariate logistic regression analysis of factors associated with baseline TMP-SMX use
Disclosures: A. Mendel, None; H. Behlouli, None; C. Moura, None; E. Vinet, None; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; S. Bernatsky, None.
Background/Purpose: Trimethoprim sulfamethoxazole (TMP-SMX) prophylaxis is recommended during induction of ANCA-associated vasculitis (AAV) to prevent pneumocystis jirovecii pneumonia (PJP), and may also reduce the risk of other serious infections. Our objective was to describe the frequency and persistence of TMP-SMX prophylaxis in patients with granulomatosis with polyangiitis (GPA) treated with rituximab (RTX), and determine factors associated with TMP-SMX use.
Methods: We identified adults meeting prior ICD case definitions for GPA from the MarketScan United States Commercial Claims and Medicaid health care databases (2011-2019). Those with a RTX claim coincident with or following the first GPA diagnostic code were included. The primary outcome was baseline TMP-SMX prophylaxis, defined as a minimum 28-day TMP-SMX prescription, overlapping with the first RTX treatment or dispensed within the following month. Covariates included age, sex, calendar year, glucocorticoid (GC) use in the 30 days prior to RTX, cyclophosphamide use, hospital admission in the 6 months prior to RTX, and baseline conditions (sinusitis, lung disease, chronic kidney disease, dialysis). We estimated the frequency and persistence of TMP-SMX use over time, allowing a prescription refill gap of 14 days. Univariable and multivariable logistic regressions were performed to identify factors associated with baseline TMP-SMX. We tested for calendar-year trends in TMP-SMX use.
Results: We studied 1921 RTX-treated GPA patients, including 222 (12%) Medicaid beneficiaries (Table 1). At the time of RTX initiation, mean age was 50 (SD 16) years, 54% were female, and median time from the first GPA diagnostic code was 67 days (IQR 16, 290). TMP-SMX was dispensed to 440 (23%) subjects at baseline. An additional 175 (9%) started TMP-SMX later in the 6-month period following RTX. TMP-SMX use increased over time, from 19% in 2011 to 28% in 2019 (p trend=0.023). Prophylaxis persisted for a median of 130 days (IQR 85, 205), with 126/440 (31%) TMP-SMX users persisting for at least 6 months. In multivariable logistic regression analyses, female sex (OR 0.68, 95% CI 0.50-0.80) was negatively associated with baseline prophylaxis, while prior hospitalization (OR 1.46, 95% CI 1.1-1.9) and GC use (OR 3.42, 95% CI 2.7-4.4) were positively associated with prophylaxis (Table 2).
Conclusion: TMP-SMX prophylaxis is dispensed to less than a quarter of GPA patients at the time of RTX, but has increased over calendar time. Recent GC had the greatest association with prophylaxis. Further work is needed to confirm if TMP-SMX reduces not only the incidence of PJP but also all-cause serious infections in patients with GPA.
 Table 1. Characteristics of GPA cohort at the time of rituximab, overall and according to baseline trimethoprim sulfamethoxazole (TMP-SMX) use
Table 1. Characteristics of GPA cohort at the time of rituximab, overall and according to baseline trimethoprim sulfamethoxazole (TMP-SMX) use Table 2. Univariate and multivariate logistic regression analysis of factors associated with baseline TMP-SMX use
Table 2. Univariate and multivariate logistic regression analysis of factors associated with baseline TMP-SMX use Disclosures: A. Mendel, None; H. Behlouli, None; C. Moura, None; E. Vinet, None; J. Curtis, Amgen, Bristol-Myers Squibb (BMS), CorEvitas, IlluminationHealth, Janssen, Lilly, Myriad, Novartis, Pfizer, Sanofi, UCB, Aqtual, Bendcare, FASTER, GlaxoSmithKlein (GSK), Labcorp, Scipher, Setpoint, United Rheumatology, AbbVie, ArthritisPower; S. Bernatsky, None.

