Back
Poster Session B
Reproductive health
Session: (0939–0969) Reproductive Issues in Rheumatic Disorders Poster
0954: What Drives the BASDAI in Pregnant Patients with Axial Spondyloarthritis? A Pooled Analysis of Four European Pregnancy Registries
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- YM
Yvette Meissner, PhD
Deutsches Rheuma-Forschungszentrum
Berlin, Germany
Abstract Poster Presenter(s)
Yvette Meissner1, Nathalie Costedoat-Chalumeau2, Rebecca Fischer-Betz3, Frauke Foerger4, Anna Molto5, Marianne Wallenius6 and Anja Strangfeld1, 1Deutsches Rheuma-Forschungszentrum Berlin, Berlin, Germany, 2Inserm DR Paris 5, Paris, France, 3Uniklinik Duesseldorf, Duesseldorf, Germany, 4Inselspital Bern, Bern, Switzerland, 5Rheumatology Department, Cochin Hospital, APHP, Paris, France, 6St. Olav University Hospital, Trondheim, Norway
Background/Purpose: The patient reported Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) includes the six components fatigue, neck, back or hip pain, pain or swelling in other joints, tenderness, morning stiffness severity and duration on a 0-10 scale. This study aims to explore the driving factors for the BASDAI in pregnant patients with axial spondyloarthritis (axSpA).
Methods: Anonymized pooled data of the European Network of Pregnancy Registries in Rheumatology (EuNeP) were used. The four participating registries are located in France, Germany, Norway and Switzerland, and collect data of women with child wish, during and after pregnancy prospectively and nationwide on regular time points. For the analysis, women who fulfilled ASAS classification criteria for axSpA and for whom a pregnancy outcome was reported until 12/2019 or 07/2020, depending on the registry, were selected. Mean BASDAI and its components were analysed descriptively.
Results: A total of 332 pregnancies from 304 women with axSpA were eligible. The Norwegian registry contributed half of the pregnancies (50.3%), followed by Germany (26.2%), France (15.4%) and Switzerland (8.1%). Mean maternal age was 31 years, the average disease duration 5 years.
Mean BASDAI was 3.0 before conception, 3.4, 3.4 and 3.5 in the 1st, 2nd and 3rd trimester, and 3.4 within 6 months postpartum. The figure shows mean values of the BASDAI and its individual components in the different time periods. Fatigue was higher than the mean score during all phases, and especially elevated in the 1st and 3rd trimester. Furthermore, values for neck, back or hip pain were higher than the mean score, especially from 2nd trimester on. All other components were lower than the mean score.
Data were not reported for all pregnancies and all time periods. Availability was highest in the 2nd and 3rd trimester with reported BASDAI in 60% and 62% of the pregnancies, respectively. Lowest reporting was 24% in the preconception period because only a part of the women was also observed before pregnancy.
Conclusion: The BASDAI is a validated instrument for assessing disease activity in patients with axSpA. Since the calculation of the score also includes factors that can be influenced by pregnancy, it may only be of limited value for measuring disease activity in pregnancy. This analysis shows that mainly fatigue and back pain in particular have an impact on the mean BASDAI. A limitation of this analysis is that data were not available for all measured time points of the individual pregnancies. Therefore, the results should be confirmed by other studies.
Acknowledgements: This work was supported by a research grant from FOREUM Foundation for Research in Rheumatology.
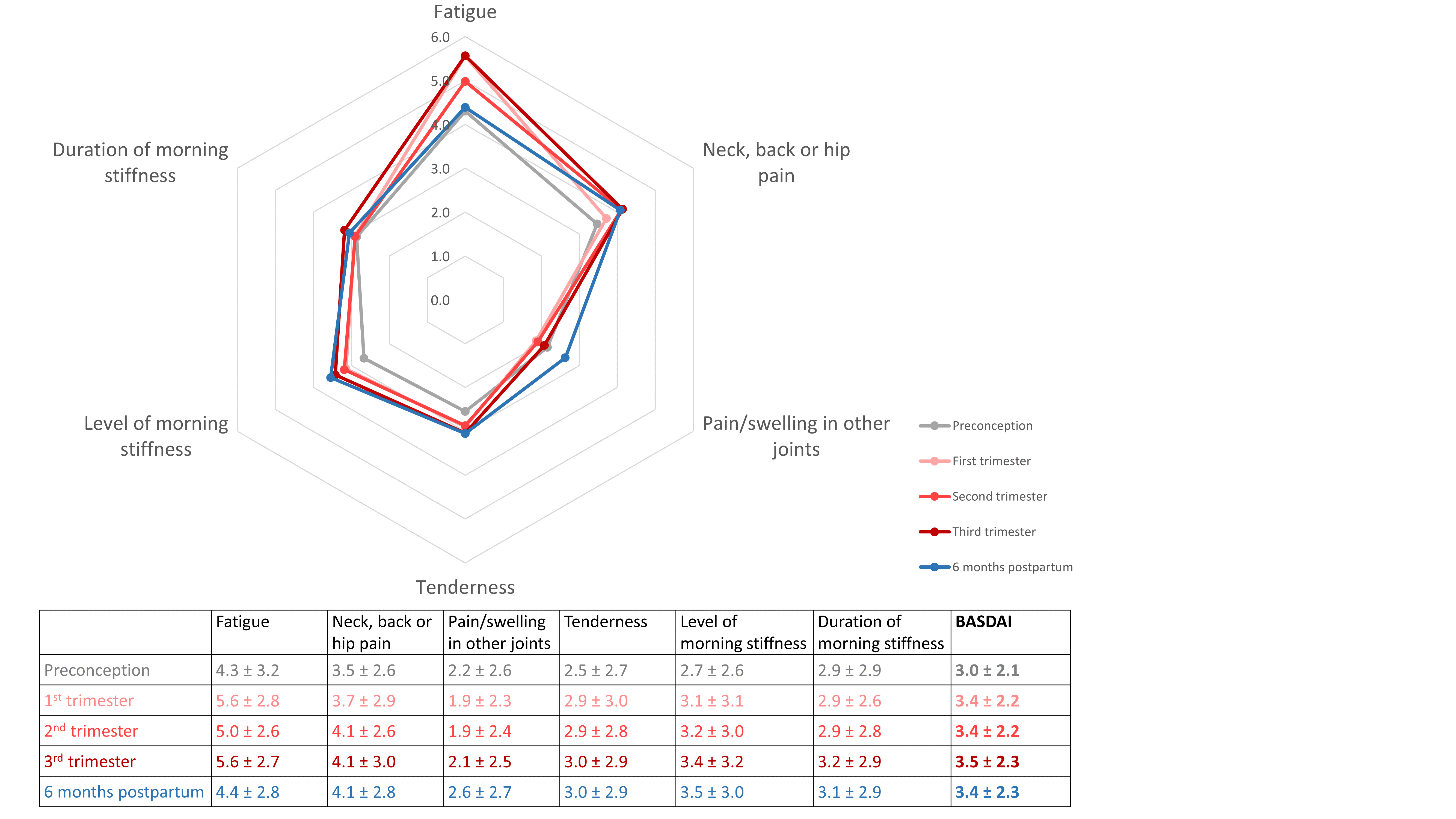 Means of BASDAI components before, during and after pregnancy (the table presents means ± standard deviation).
Means of BASDAI components before, during and after pregnancy (the table presents means ± standard deviation).
Disclosures: Y. Meissner, Pfizer; N. Costedoat-Chalumeau, UCB, Roche; R. Fischer-Betz, None; F. Foerger, UCB Pharma, GlaxoSmithKlein(GSK), Roche; A. Molto, None; M. Wallenius, None; A. Strangfeld, AbbVie/Abbott, Merck/MSD, Roche, Bristol-Myers Squibb(BMS), Pfizer.
Background/Purpose: The patient reported Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) includes the six components fatigue, neck, back or hip pain, pain or swelling in other joints, tenderness, morning stiffness severity and duration on a 0-10 scale. This study aims to explore the driving factors for the BASDAI in pregnant patients with axial spondyloarthritis (axSpA).
Methods: Anonymized pooled data of the European Network of Pregnancy Registries in Rheumatology (EuNeP) were used. The four participating registries are located in France, Germany, Norway and Switzerland, and collect data of women with child wish, during and after pregnancy prospectively and nationwide on regular time points. For the analysis, women who fulfilled ASAS classification criteria for axSpA and for whom a pregnancy outcome was reported until 12/2019 or 07/2020, depending on the registry, were selected. Mean BASDAI and its components were analysed descriptively.
Results: A total of 332 pregnancies from 304 women with axSpA were eligible. The Norwegian registry contributed half of the pregnancies (50.3%), followed by Germany (26.2%), France (15.4%) and Switzerland (8.1%). Mean maternal age was 31 years, the average disease duration 5 years.
Mean BASDAI was 3.0 before conception, 3.4, 3.4 and 3.5 in the 1st, 2nd and 3rd trimester, and 3.4 within 6 months postpartum. The figure shows mean values of the BASDAI and its individual components in the different time periods. Fatigue was higher than the mean score during all phases, and especially elevated in the 1st and 3rd trimester. Furthermore, values for neck, back or hip pain were higher than the mean score, especially from 2nd trimester on. All other components were lower than the mean score.
Data were not reported for all pregnancies and all time periods. Availability was highest in the 2nd and 3rd trimester with reported BASDAI in 60% and 62% of the pregnancies, respectively. Lowest reporting was 24% in the preconception period because only a part of the women was also observed before pregnancy.
Conclusion: The BASDAI is a validated instrument for assessing disease activity in patients with axSpA. Since the calculation of the score also includes factors that can be influenced by pregnancy, it may only be of limited value for measuring disease activity in pregnancy. This analysis shows that mainly fatigue and back pain in particular have an impact on the mean BASDAI. A limitation of this analysis is that data were not available for all measured time points of the individual pregnancies. Therefore, the results should be confirmed by other studies.
Acknowledgements: This work was supported by a research grant from FOREUM Foundation for Research in Rheumatology.
 Means of BASDAI components before, during and after pregnancy (the table presents means ± standard deviation).
Means of BASDAI components before, during and after pregnancy (the table presents means ± standard deviation). Disclosures: Y. Meissner, Pfizer; N. Costedoat-Chalumeau, UCB, Roche; R. Fischer-Betz, None; F. Foerger, UCB Pharma, GlaxoSmithKlein(GSK), Roche; A. Molto, None; M. Wallenius, None; A. Strangfeld, AbbVie/Abbott, Merck/MSD, Roche, Bristol-Myers Squibb(BMS), Pfizer.

