Back
Abstract Session
Diversity, inclusion and racial disparities
Session: Abstracts: Healthcare Disparities in Rheumatology (1098–1101)
1100: Racial Differences in Clinical Trial Perceptions Among a Large, Predominantly Black Cohort of Patients with Systemic Lupus Erythematosus in the Southeastern United States
Sunday, November 13, 2022
11:00 AM – 11:10 AM Eastern Time
Location: Room 120

Jessica Williams, MD, MPH
Emory University School of Medicine
Atlanta, GA, United States
Presenting Author(s)
Jessica Williams1, Gaobin Bao1, Charmayne Dunlop-Thomas2, Cristina Drenkard2, Kim Schofield1 and S. Sam Lim2, 1Emory University School of Medicine, Atlanta, GA, 2Emory University, Atlanta, GA
Background/Purpose: Black patients have higher incidence and severity of systemic lupus erythematosus (SLE) and worse outcomes as compared to White patients, yet Black patients are significantly underrepresented in SLE clinical trials. We assessed racial differences in clinical trial perceptions among the largest cohort of predominantly Black patients with SLE ever assembled in the United States.
Methods: Georgians Organized Against Lupus (GOAL) is a prospective cohort of validated patients with SLE living in Atlanta. Participants have been surveyed annually since 2012 regarding demographics, SLE natural history, treatment, healthcare utilization, and psychosocial factors. The 2021 GOAL survey included questions assessing clinical trial knowledge, trustworthy sources of trial information, prior experience with trial recruitment/participation, willingness to participate in trials, and the impact of race and gender on participation. Self-reported race was categorized as Black or Non-Black. Survey responses by race were compared using Chi-squared analyses. Among Black patients, factors associated with willingness to participate in clinical trials were examined using univariable logistic regression.
Results: A total of 708 individuals responded to the 2021 GOAL survey, of whom 80% were Black. Among the remaining 20%, 88% were White, 11% were Asian, and 1% were another race. Compared to non-Black respondents, Black respondents were significantly less likely to correctly identify the definition of a clinical trial (34% vs. 72%, p< 0.001) and less likely to trust their rheumatologist about clinical trial information (90 vs. 96%, p=0.034); Table 1. Black respondents were significantly more likely to trust their lupus support group about clinical trial information (26% vs. 11%, p< 0.001) and to prefer clinical trials involving study staff with racial (24% vs. 10%, p< 0.001) and gender concordance (21% vs. 14%, p< 0.001), as well as trials targeting the respondent's racial (30% vs. 12%, p< 0.001) or gender group (26% vs. 20%, p< 0.001). There was no significant difference in willingness to participate in clinical trials between Black and non-Black respondents (28% vs. 30%, p=0.10). Younger age, male sex, unemployed or disabled status, federal health insurance, higher disease activity, and severe damage were associated with willingness to participate in clinical trials among Black respondents (Table 2).
Conclusion: We found that only about 30% of respondents were willing to participate in lupus clinical trials, with similar willingness to participate among Black and non-Black respondents. Efforts must continue to engage those resistant to trial participation. Our findings also indicate that clinical trial education, recruitment through lupus support groups, increased racial and gender diversity of study staff, and race- and gender-specific trials are potential strategies to increase Black patient recruitment. Age, sex, work status, insurance status, and lupus activity and damage may also play important roles in clinical trial participation among Black patients. Further studies are needed to validate these findings in other large populations of Black patients with SLE.
.jpg)
.jpg)
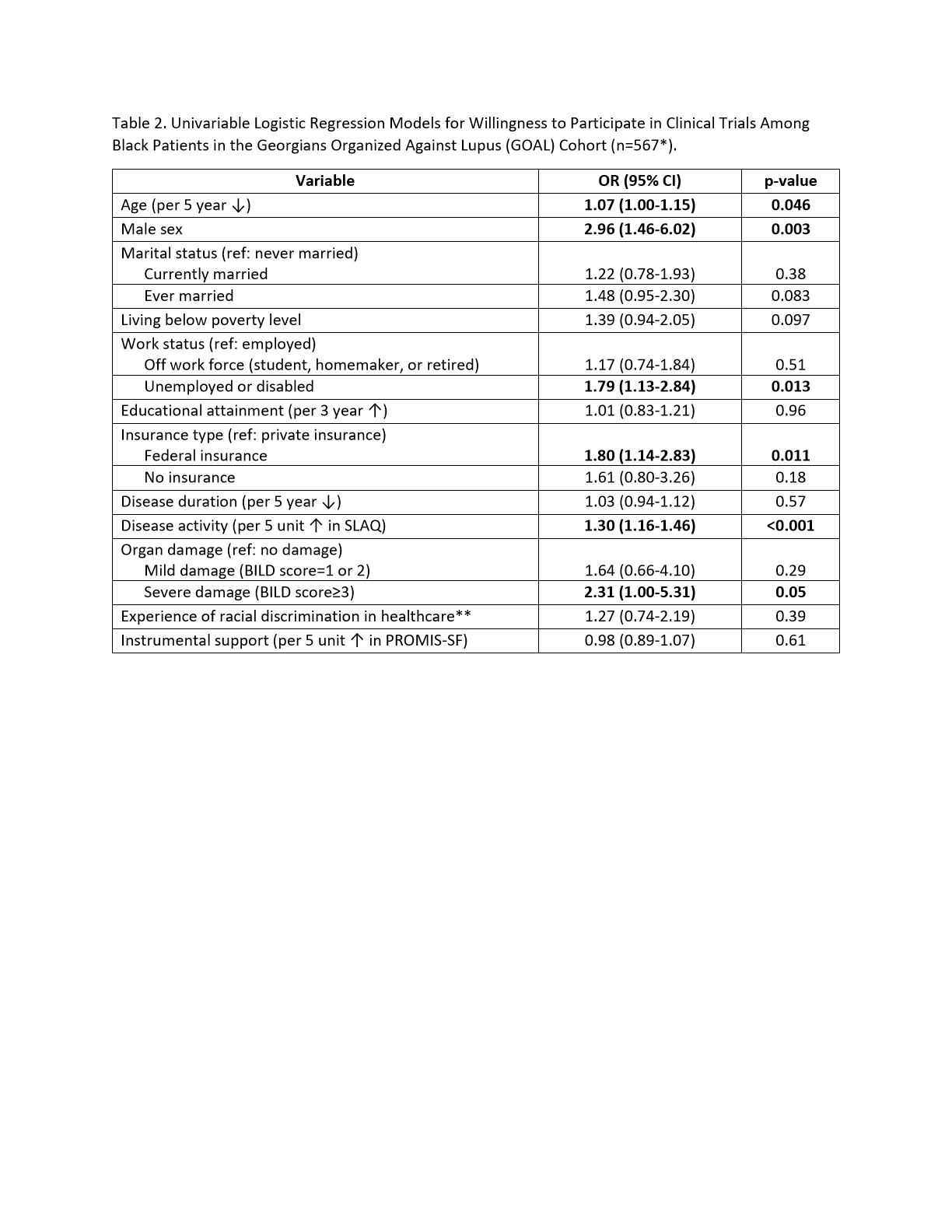 *Two patients excluded due to missing responses. **Defined as a response of always, usually, or sometimes on the IPC-29: Interpersonal Processes of Care Survey. OR=odds ratio; CI=confidence interval; ref=reference; SLAQ=Systemic Lupus Activity Questionnaire; BILD=Brief Index of Lupus Damage; PROMIS-SF= Patient Reported Outcome Measurement Information System-Short Form.
*Two patients excluded due to missing responses. **Defined as a response of always, usually, or sometimes on the IPC-29: Interpersonal Processes of Care Survey. OR=odds ratio; CI=confidence interval; ref=reference; SLAQ=Systemic Lupus Activity Questionnaire; BILD=Brief Index of Lupus Damage; PROMIS-SF= Patient Reported Outcome Measurement Information System-Short Form.
Disclosures: J. Williams, None; G. Bao, None; C. Dunlop-Thomas, None; C. Drenkard, None; K. Schofield, None; S. Lim, None.
Background/Purpose: Black patients have higher incidence and severity of systemic lupus erythematosus (SLE) and worse outcomes as compared to White patients, yet Black patients are significantly underrepresented in SLE clinical trials. We assessed racial differences in clinical trial perceptions among the largest cohort of predominantly Black patients with SLE ever assembled in the United States.
Methods: Georgians Organized Against Lupus (GOAL) is a prospective cohort of validated patients with SLE living in Atlanta. Participants have been surveyed annually since 2012 regarding demographics, SLE natural history, treatment, healthcare utilization, and psychosocial factors. The 2021 GOAL survey included questions assessing clinical trial knowledge, trustworthy sources of trial information, prior experience with trial recruitment/participation, willingness to participate in trials, and the impact of race and gender on participation. Self-reported race was categorized as Black or Non-Black. Survey responses by race were compared using Chi-squared analyses. Among Black patients, factors associated with willingness to participate in clinical trials were examined using univariable logistic regression.
Results: A total of 708 individuals responded to the 2021 GOAL survey, of whom 80% were Black. Among the remaining 20%, 88% were White, 11% were Asian, and 1% were another race. Compared to non-Black respondents, Black respondents were significantly less likely to correctly identify the definition of a clinical trial (34% vs. 72%, p< 0.001) and less likely to trust their rheumatologist about clinical trial information (90 vs. 96%, p=0.034); Table 1. Black respondents were significantly more likely to trust their lupus support group about clinical trial information (26% vs. 11%, p< 0.001) and to prefer clinical trials involving study staff with racial (24% vs. 10%, p< 0.001) and gender concordance (21% vs. 14%, p< 0.001), as well as trials targeting the respondent's racial (30% vs. 12%, p< 0.001) or gender group (26% vs. 20%, p< 0.001). There was no significant difference in willingness to participate in clinical trials between Black and non-Black respondents (28% vs. 30%, p=0.10). Younger age, male sex, unemployed or disabled status, federal health insurance, higher disease activity, and severe damage were associated with willingness to participate in clinical trials among Black respondents (Table 2).
Conclusion: We found that only about 30% of respondents were willing to participate in lupus clinical trials, with similar willingness to participate among Black and non-Black respondents. Efforts must continue to engage those resistant to trial participation. Our findings also indicate that clinical trial education, recruitment through lupus support groups, increased racial and gender diversity of study staff, and race- and gender-specific trials are potential strategies to increase Black patient recruitment. Age, sex, work status, insurance status, and lupus activity and damage may also play important roles in clinical trial participation among Black patients. Further studies are needed to validate these findings in other large populations of Black patients with SLE.
.jpg)
.jpg)
 *Two patients excluded due to missing responses. **Defined as a response of always, usually, or sometimes on the IPC-29: Interpersonal Processes of Care Survey. OR=odds ratio; CI=confidence interval; ref=reference; SLAQ=Systemic Lupus Activity Questionnaire; BILD=Brief Index of Lupus Damage; PROMIS-SF= Patient Reported Outcome Measurement Information System-Short Form.
*Two patients excluded due to missing responses. **Defined as a response of always, usually, or sometimes on the IPC-29: Interpersonal Processes of Care Survey. OR=odds ratio; CI=confidence interval; ref=reference; SLAQ=Systemic Lupus Activity Questionnaire; BILD=Brief Index of Lupus Damage; PROMIS-SF= Patient Reported Outcome Measurement Information System-Short Form.Disclosures: J. Williams, None; G. Bao, None; C. Dunlop-Thomas, None; C. Drenkard, None; K. Schofield, None; S. Lim, None.

