Back
Plenary Session
Systemic lupus erythematosus (SLE)
Session: Plenary II
1117: Efficacy and Safety of Deucravacitinib, an Oral, Selective, Allosteric TYK2 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study
Sunday, November 13, 2022
12:45 PM – 12:55 PM Eastern Time
Location: Exhibit Hall A
- MP
Marilyn Pike, MD, PhD
MedPharm consulting, Inc.
RALEIGH, NC, United States
Presenting Author(s)
Marilyn Pike1, Joan Merrill2, Eric Morand3, Ronald van Vollenhoven4, Victoria Werth5, Coburn Hobar6, Nikolay Delev6, Vaishali Shah7, Brian Sharkey6, Thomas Wegman6, Ian M. Catlett6, Subhashis Banerjee6 and Shalabh Singhal6, 1MedPharm Consulting Inc., Raleigh, NC, 2Oklahoma Medical Research Foundation, Oklahoma City, OK, 3Monash University, Victoria; Department of Rheumatology, Monash Health, Melbourne, Australia, 4Amsterdam University Medical Centers, Amsterdam, Netherlands, 5University of Pennsylvania, Philadelphia, PA, 6Bristol Myers Squibb, Princeton, NJ, 7Bristol Myers Squibb, Robbinsville, NJ
Background/Purpose: Tyrosine kinase 2 (TYK2) mediates signaling of Type I IFNs, IL-23, and IL-12, key cytokines involved in lupus pathogenesis. Deucravacitinib (DEUC) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action, distinct from Janus kinase (JAK) 1/2/3 inhibitors, and has shown efficacy in psoriasis and psoriatic arthritis. This analysis assessed the efficacy and safety of DEUC in patients with active SLE.
Methods: This was a 48-week (wk), randomized, double-blind, placebo (PBO)-controlled, phase 2 trial (NCT03252587). Eligible patients met the SLICC criteria, were seropositive (ANA/anti-dsDNA/anti-Sm), and had a SLEDAI-2K score ≥ 6 and ≥ 1 BILAG index A or > 2 BILAG B manifestations from the musculoskeletal or mucocutaneous domain. Patients on standard background medications were randomized 1:1:1:1 to PBO or DEUC (3 mg BID, 6 mg BID, or 12 mg QD). Oral corticosteroid tapering to 7.5 mg/day was required from wks 8-20; further tapering was optional from wks 32-40. The primary endpoint was the proportion of patients achieving SLE responder index (SRI[4]) at wk 32. Key secondary endpoints at wk 48 included SRI(4), BILAG-based Composite Lupus Assessment (BICLA), Lupus Low Disease Activity State (LLDAS), decrease of ≥ 50% from baseline Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI-50), and change from baseline in active (tender and swollen) joint count.
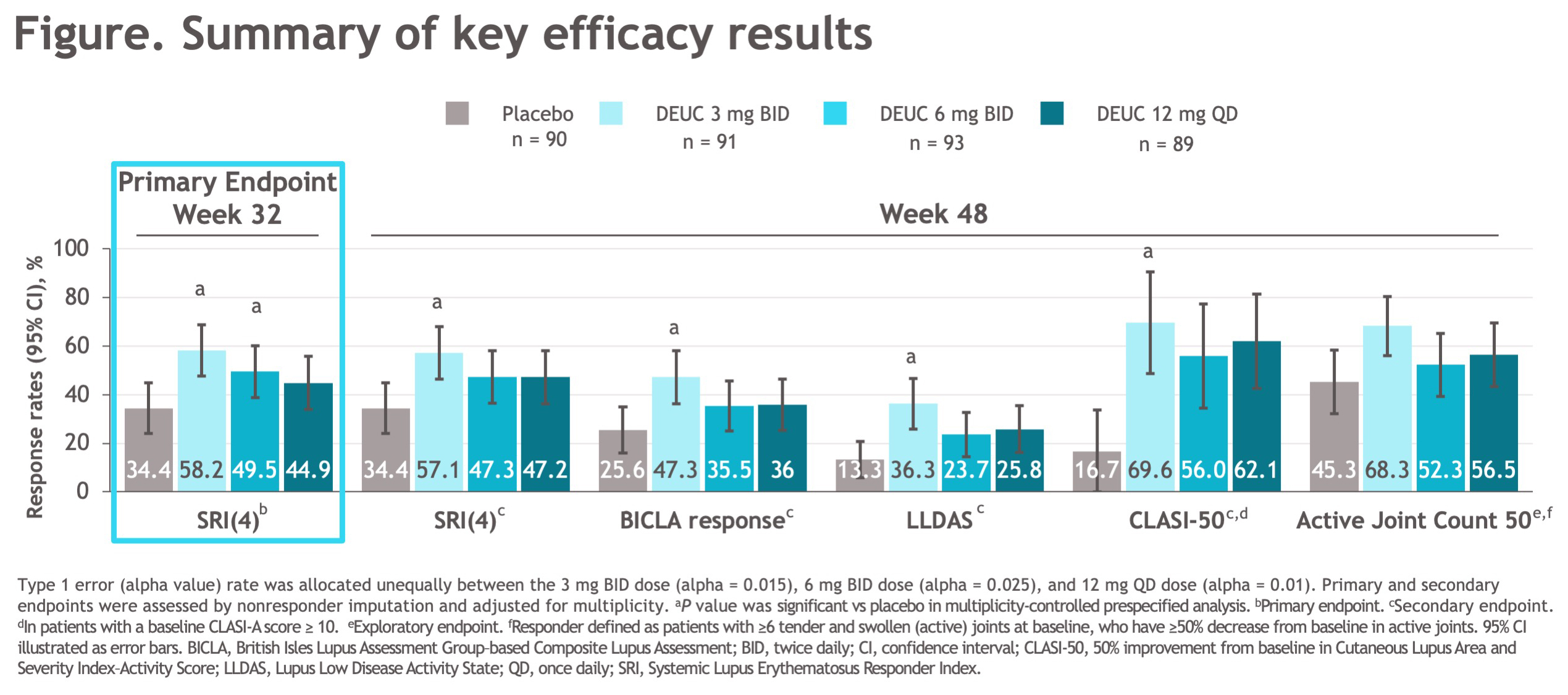
Results: A total of 363 patients were randomized, with baseline demographic and disease characteristics similar across treatment groups. Of randomized patients, 275 (76%) completed 48 wks of treatment. The primary endpoint at wk 32 was met, with significantly greater proportion of patients in DEUC 3 mg BID and 6 mg BID groups vs PBO achieving SRI(4) responses (PBO: 34.4%; DEUC 3 mg BID: 58.2%, P=0.0006; DEUC 6 mg BID: 49.5%, P=0.021; DEUC 12 mg QD: 44.9%, P=0.078). SRI(4) response was sustained across all DEUC groups up to 48 wks (Figure). At wk 48, the DEUC 3 mg BID group demonstrated statistical significance in BICLA, LLDAS, CLASI-50, and active joint count, and the two other DEUC groups demonstrated clinically meaningful differences vs PBO (Figure). Rates of adverse events (AEs), serious AEs, and AEs of interest were similar between DEUC and PBO groups (Table). Most common AEs (≥10%) with DEUC were upper respiratory tract infection, nasopharyngitis, headache, and urinary tract infection. No deaths, major cardiac events, thrombotic events, systemic opportunistic infections, or active tuberculosis occurred. Malignancies were rare with similar rates across all groups. No meaningful abnormalities in mean levels of hematology and chemistry laboratory parameters were observed.
Conclusion: In patients with active SLE, DEUC showed statistically significant and sustained clinical efficacy in SRI(4) responses versus PBO and was well tolerated. All secondary endpoints were achieved or meaningfully improved with DEUC treatment at wk 48, including SRI(4), BICLA, LLDAS, CLASI-50, and change in active joint count. DEUC shows promise as a novel therapy for SLE and warrants further investigation in phase 3 trials.
.jpg)
Disclosures: M. Pike, AstraZeneca, Bristol Myers Squibb, Pfizer; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; V. Werth, Celgene, Medimmune, Resolve, Genentech, Idera, Janssen, Lilly, Biogen, Bristol Myers Squibb, Gilead, Amgen, Medscape, Nektar, Incyte, EMD Serono, CSL Behring, Principia, Crisalis, Viela Bio, Argenx, Kirin, AstraZeneca, AbbVie, GSK, Cugene, UCB, Beacon Bioscience, Corcept, Gilead, Lupus Research Alliance/BMS; C. Hobar, Bristol Myers Squibb; N. Delev, Bristol-Myers Squibb(BMS); V. Shah, Bristol Myers Squibb; B. Sharkey, Bristol Myers Squibb; T. Wegman, Bristol Myers Squibb; I. Catlett, Bristol Myers Squibb; S. Banerjee, Bristol Myers Squibb; S. Singhal, Bristol Myers Squibb.
Background/Purpose: Tyrosine kinase 2 (TYK2) mediates signaling of Type I IFNs, IL-23, and IL-12, key cytokines involved in lupus pathogenesis. Deucravacitinib (DEUC) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action, distinct from Janus kinase (JAK) 1/2/3 inhibitors, and has shown efficacy in psoriasis and psoriatic arthritis. This analysis assessed the efficacy and safety of DEUC in patients with active SLE.
Methods: This was a 48-week (wk), randomized, double-blind, placebo (PBO)-controlled, phase 2 trial (NCT03252587). Eligible patients met the SLICC criteria, were seropositive (ANA/anti-dsDNA/anti-Sm), and had a SLEDAI-2K score ≥ 6 and ≥ 1 BILAG index A or > 2 BILAG B manifestations from the musculoskeletal or mucocutaneous domain. Patients on standard background medications were randomized 1:1:1:1 to PBO or DEUC (3 mg BID, 6 mg BID, or 12 mg QD). Oral corticosteroid tapering to 7.5 mg/day was required from wks 8-20; further tapering was optional from wks 32-40. The primary endpoint was the proportion of patients achieving SLE responder index (SRI[4]) at wk 32. Key secondary endpoints at wk 48 included SRI(4), BILAG-based Composite Lupus Assessment (BICLA), Lupus Low Disease Activity State (LLDAS), decrease of ≥ 50% from baseline Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI-50), and change from baseline in active (tender and swollen) joint count.
Results: A total of 363 patients were randomized, with baseline demographic and disease characteristics similar across treatment groups. Of randomized patients, 275 (76%) completed 48 wks of treatment. The primary endpoint at wk 32 was met, with significantly greater proportion of patients in DEUC 3 mg BID and 6 mg BID groups vs PBO achieving SRI(4) responses (PBO: 34.4%; DEUC 3 mg BID: 58.2%, P=0.0006; DEUC 6 mg BID: 49.5%, P=0.021; DEUC 12 mg QD: 44.9%, P=0.078). SRI(4) response was sustained across all DEUC groups up to 48 wks (Figure). At wk 48, the DEUC 3 mg BID group demonstrated statistical significance in BICLA, LLDAS, CLASI-50, and active joint count, and the two other DEUC groups demonstrated clinically meaningful differences vs PBO (Figure). Rates of adverse events (AEs), serious AEs, and AEs of interest were similar between DEUC and PBO groups (Table). Most common AEs (≥10%) with DEUC were upper respiratory tract infection, nasopharyngitis, headache, and urinary tract infection. No deaths, major cardiac events, thrombotic events, systemic opportunistic infections, or active tuberculosis occurred. Malignancies were rare with similar rates across all groups. No meaningful abnormalities in mean levels of hematology and chemistry laboratory parameters were observed.
Conclusion: In patients with active SLE, DEUC showed statistically significant and sustained clinical efficacy in SRI(4) responses versus PBO and was well tolerated. All secondary endpoints were achieved or meaningfully improved with DEUC treatment at wk 48, including SRI(4), BICLA, LLDAS, CLASI-50, and change in active joint count. DEUC shows promise as a novel therapy for SLE and warrants further investigation in phase 3 trials.

.jpg)
Disclosures: M. Pike, AstraZeneca, Bristol Myers Squibb, Pfizer; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; E. Morand, AbbVie/Abbott, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb(BMS), Eli Lilly, Genentech, GlaxoSmithKlein(GSK), Janssen, Novartis, Servier, UCB, EMD Serono, Billerica, MA, USA; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; V. Werth, Celgene, Medimmune, Resolve, Genentech, Idera, Janssen, Lilly, Biogen, Bristol Myers Squibb, Gilead, Amgen, Medscape, Nektar, Incyte, EMD Serono, CSL Behring, Principia, Crisalis, Viela Bio, Argenx, Kirin, AstraZeneca, AbbVie, GSK, Cugene, UCB, Beacon Bioscience, Corcept, Gilead, Lupus Research Alliance/BMS; C. Hobar, Bristol Myers Squibb; N. Delev, Bristol-Myers Squibb(BMS); V. Shah, Bristol Myers Squibb; B. Sharkey, Bristol Myers Squibb; T. Wegman, Bristol Myers Squibb; I. Catlett, Bristol Myers Squibb; S. Banerjee, Bristol Myers Squibb; S. Singhal, Bristol Myers Squibb.

