Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1564: Anti-TNF versus Anti-IL6 Receptor Antagonist Therapy in Severe Ocular Involvement in Behçet's Disease
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NB
Nuria Barroso Garcia, -None-
Hospital Universitario Puerta del Mar
Cadiz, Spain
Abstract Poster Presenter(s)
Nuria Barroso-García1, Belén Atienza-Mateo2, Ivan Ferraz Amaro3, Diana Prieto-Peña2, Ana De Vicente-Delmás4, Ricardo Blanco5 and Miguel Ángel González-Gay6, 1Hospital Universitario Puerta del Mar, Cádiz, Spain, Cádiz, Spain, 2Research Group on Genetic Epidemiology and Atherosclerosis in Systemic Diseases and in Metabolic Bone Diseases of the Musculoskeletal System, IDIVAL; and Department of Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Division of Rheumatology. Hospital Universitario de Canarias. Spain., Santa Cruz de Tenerife, Spain, 4Hospital General Santa María del Puerto, Cádiz, Spain, 5Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 6Department of Medicine and Psychiatry, Universidad de Cantabria; Rheumatology Division, Hospital Universitario Marqués de Valdecilla; Research group on genetic epidemiology and atherosclerosis in systemic diseases and in metabolic diseases of the musculoskeletal system, IDIVAL, Santander, Spain. Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Background/Purpose: To evaluate the efficacy of anti-TNF or anti-IL6 treatment in severe and refractory ocular involvement in patients with refractory cystoid macular edema (CME) due to Behçet's disease (BD).
Methods: Multicenter study of patients with BD-associated ocular involvement refractory to immunosuppressive drugs. Patients with CME associated to BD uveitis were selected from a cohort of 177 patients treated with Infliximab (IFX) and/or Adalimumab (ADA) and 14 patients treated with Tocilizumab (TCZ). From baseline up to 4 years of follow-up, the evolution of macular thickness (main outcome), best-corrected visual acuity (BCVA) and intraocular inflammation (Tyndall and vitritis) was analyzed.
Results: A total of 49 patients with CME associated BD (and 72 eyes) were included. 25 patients (40 eyes) were treated with ADA, 15 patients (21 eyes) received IFX and 9 patients (11 eyes) were treated with TCZ. There was a lower basal BCVA in patients treated with TCZ. No statistically significant baseline differences were observed in the rest of parameters. Most patients from all groups had received several conventional immunosuppressive drugs and in addition, the group of TCZ had also received anti-TNF agents. Monotherapy with biological immunosuppressive drugs was used in 8 patients and combined therapy with conventional immunosuppressive drugs was used in 41 patients (Table 1). Regarding the evolution of ocular parameters after 1 year of treatment, macular thickness progressively decreased in the 3 groups, with no signs of CME. Similarly, visual acuity and inflammatory intraocular remission improved in all groups (Figure 1).
Conclusion: Refractory CME associated with BD uveitis can be effectively treated either with ADA, IFX or TCZ. Moreover, TCZ may be an appropriate therapeutic option for patients with contraindications or resistance to anti-TNF therapy.
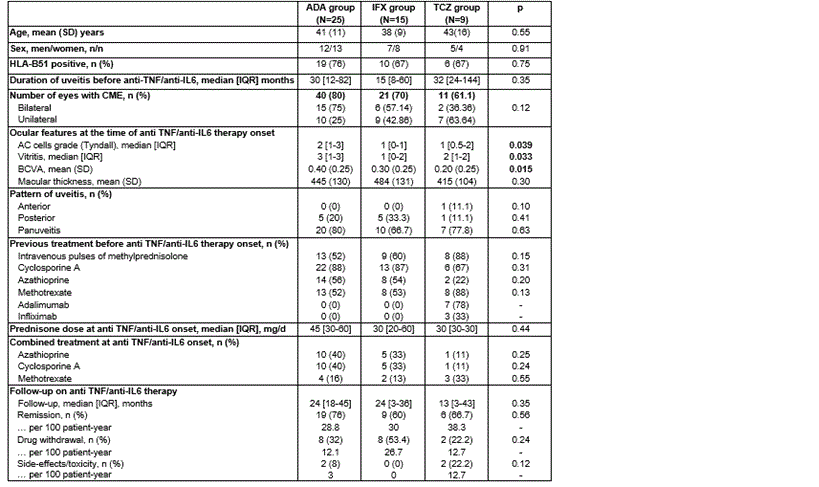 Table 1. Demographic and clinical characteristics of 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ).
Table 1. Demographic and clinical characteristics of 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ).
.gif) Figure 1. Evolution of ocular parameters in 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ).
Figure 1. Evolution of ocular parameters in 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ).
Disclosures: N. Barroso-García, None; B. Atienza-Mateo, AbbVie/Abbott, Roche, Pfizer, Celgene, Novartis, Janssen, UCB, Eli Lilly; I. Ferraz Amaro, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Pfizer, Roche, Amgen, Celgene, Merck/MSD; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; A. De Vicente-Delmás, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD.
Background/Purpose: To evaluate the efficacy of anti-TNF or anti-IL6 treatment in severe and refractory ocular involvement in patients with refractory cystoid macular edema (CME) due to Behçet's disease (BD).
Methods: Multicenter study of patients with BD-associated ocular involvement refractory to immunosuppressive drugs. Patients with CME associated to BD uveitis were selected from a cohort of 177 patients treated with Infliximab (IFX) and/or Adalimumab (ADA) and 14 patients treated with Tocilizumab (TCZ). From baseline up to 4 years of follow-up, the evolution of macular thickness (main outcome), best-corrected visual acuity (BCVA) and intraocular inflammation (Tyndall and vitritis) was analyzed.
Results: A total of 49 patients with CME associated BD (and 72 eyes) were included. 25 patients (40 eyes) were treated with ADA, 15 patients (21 eyes) received IFX and 9 patients (11 eyes) were treated with TCZ. There was a lower basal BCVA in patients treated with TCZ. No statistically significant baseline differences were observed in the rest of parameters. Most patients from all groups had received several conventional immunosuppressive drugs and in addition, the group of TCZ had also received anti-TNF agents. Monotherapy with biological immunosuppressive drugs was used in 8 patients and combined therapy with conventional immunosuppressive drugs was used in 41 patients (Table 1). Regarding the evolution of ocular parameters after 1 year of treatment, macular thickness progressively decreased in the 3 groups, with no signs of CME. Similarly, visual acuity and inflammatory intraocular remission improved in all groups (Figure 1).
Conclusion: Refractory CME associated with BD uveitis can be effectively treated either with ADA, IFX or TCZ. Moreover, TCZ may be an appropriate therapeutic option for patients with contraindications or resistance to anti-TNF therapy.
 Table 1. Demographic and clinical characteristics of 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ).
Table 1. Demographic and clinical characteristics of 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ)..gif) Figure 1. Evolution of ocular parameters in 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ).
Figure 1. Evolution of ocular parameters in 49 patients with cystoid macular edema due to Behçet’s disease receiving adalimumab (ADA), infliximab (IFX) or tocilizumab (TCZ). Disclosures: N. Barroso-García, None; B. Atienza-Mateo, AbbVie/Abbott, Roche, Pfizer, Celgene, Novartis, Janssen, UCB, Eli Lilly; I. Ferraz Amaro, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Pfizer, Roche, Amgen, Celgene, Merck/MSD; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; A. De Vicente-Delmás, None; R. Blanco, Eli Lilly, Pfizer, Roche, Janssen, MSD, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Galapagos, Novartis, Sanofi; M. González-Gay, AbbVie/Abbott, Merck/MSD, Janssen, Roche, AbbVie/Abbott, Roche, Sanofi, Eli Lilly, Celgene, Sobi, Merck/MSD.

