Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1186–1214) Epidemiology and Public Health Poster II
1201: Autoimmune Serologies, Cell-Bound Complement Activation Products, and Autoimmune Rheumatic Disease Symptoms After COVID-19 Infection
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- EO
Emily G. Oakes, BA
Brigham and Women's Hospital
Boxford, MA, United States
Abstract Poster Presenter(s)
Emily G Oakes1, Jack Ellrodt1, Roberta Alexander2, John Conklin2, May Choi3, Hongshu Guan1, Sara Tedeschi1, Jeffrey Sparks4, Siobhan Case5, Tiffany Hsu1, Daniel Solomon1, Anna Jonsson1 and Karen Costenbader1, 1Brigham and Women's Hospital, Boston, MA, 2Exagen, Inc., Vista, CA, 3Brigham and Women's Hospital | University of Calgary, Calgary, AB, Canada, 4Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 5Brigham and Women's Hospital, Boston Children's Hospital, Boston, MA
Background/Purpose: Onset of autoimmune connective tissue disease (CTD) after COVID-19 infection has been reported and complement activation has been implicated. We examined common autoimmune rheumatic disease symptoms, serologies, and complement activation arising after COVID-19 infection.
Methods: We conducted a cross-sectional prospective study of patients who tested positive for COVID-19 from 3/1/20-9/1/21 at a large multihospital system. Eligible patients, ≥18 years old and ≥3 months post-COVID-19 infection, received the CTD Screening Questionnaire (CSQ; Karlson EW, 1995) and the Brief Pain Inventory (BPI) by electronic or physical mail. Subjects who returned completed questionnaires, had evidence of new CTD symptoms on CSQ, and had no prior CTD diagnosis (CSQ+) were invited for a physician exam and blood draw to test for autoimmune serologies and cell-bound complement activation products (EC4d, BC4d, and PC4d, together CB-CAPs). The multi-analyte assay panel (MAP) was determined using a two-tier algorithm. Targeting equal numbers of subjects who screened positive and negative on the CSQ, we invited age- and sex-matched subjects who had COVID-19, negative CSQs, and no prior CTD diagnosis (CSQ-) for blood collection. We tested for associations between CSQ positivity after COVID-19 and autoantibodies and CB-CAPs by Fisher exact test.
Results: We invited 101 eligible CSQ+ subjects and 122 CSQ- subjects to participate: 19 CSQ+ and 22 matched CSQ- subjects were enrolled. Age and sex were well-matched between the two groups (overall mean age 53.2 [SD 13.4], 82.9% female, 73.2% white). At time of infection, 4.9% of subjects were vaccinated and 14.6% were hospitalized (Table 1). Mean pain severity and interference scores were comparable among the CSQ+ and CSQ-. Among CSQ+ subjects, 11 subjects (57.9%) screened positive for symptoms of systemic lupus erythematosus (SLE), 8 (42.1%) screened positive for symptoms of rheumatoid arthritis (RA), and 2 (10.5%) screened positive for symptoms of scleroderma (SSc). Joint pain (36.8%), shortness of breath (36.8%), and other (non malar) rash (42.1%) were the most common subject-reported new onset CTD symptoms (Table 2). On exam, 4 cases of new joint pain and 4 cases of new non malar rash were found. There was an overall low prevalence of CTD autoantibodies and no positive CB-CAPs. No significant associations were identified between new CTD symptoms by CSQ and autoantibodies, CB-CAPs, or MAP (Table 3).
Conclusion: In this comparison of subjects following COVID-19 infection with or without self-reported new CTD symptoms, we found overall low prevalence of CTD symptoms on exam and CTD-related autoantibodies, and no associations between new CTD symptoms or physical exam findings and CTD autoantibodies, CB-CAPs, or MAP positivity. To our knowledge, this is the first study of CB-CAPs, a novel complement-based biomarker for SLE, in the context of COVID-19, which is thought to activate complement. These findings suggest that CSQ symptom positivity ≥3 months post-COVID-19 infection may not be associated with formation of CB-CAPs or CTD-associated autoantibodies.
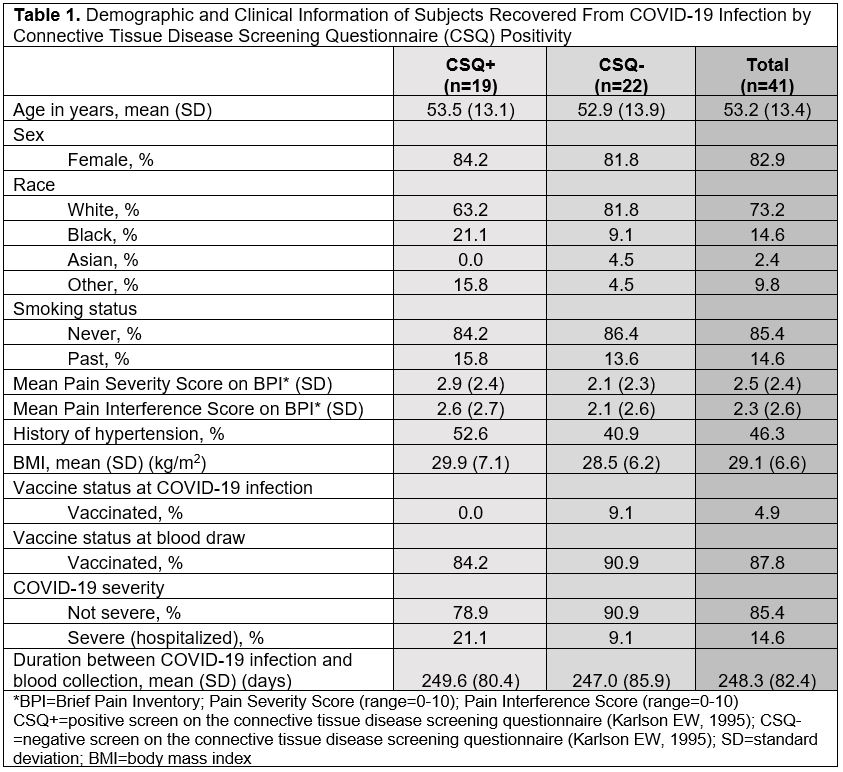 Table 1. Demographic and Clinical Information of Subjects Recovered From COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) Positivity
Table 1. Demographic and Clinical Information of Subjects Recovered From COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) Positivity
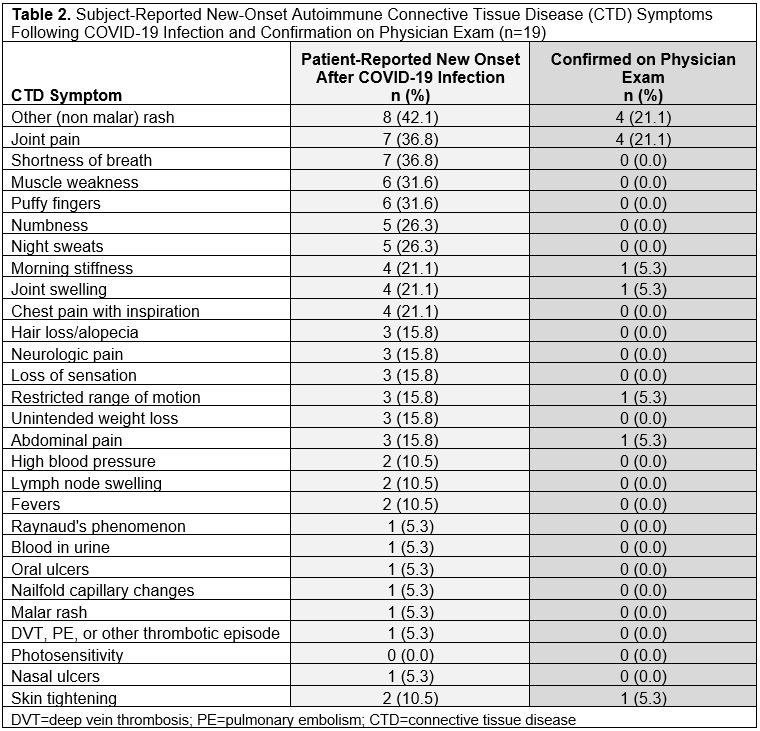 Table 2. Subject-Reported New-Onset Autoimmune Connective Tissue Disease (CTD) Symptoms Following COVID-19 Infection and Confirmation on Physician Exam (n=19)
Table 2. Subject-Reported New-Onset Autoimmune Connective Tissue Disease (CTD) Symptoms Following COVID-19 Infection and Confirmation on Physician Exam (n=19)
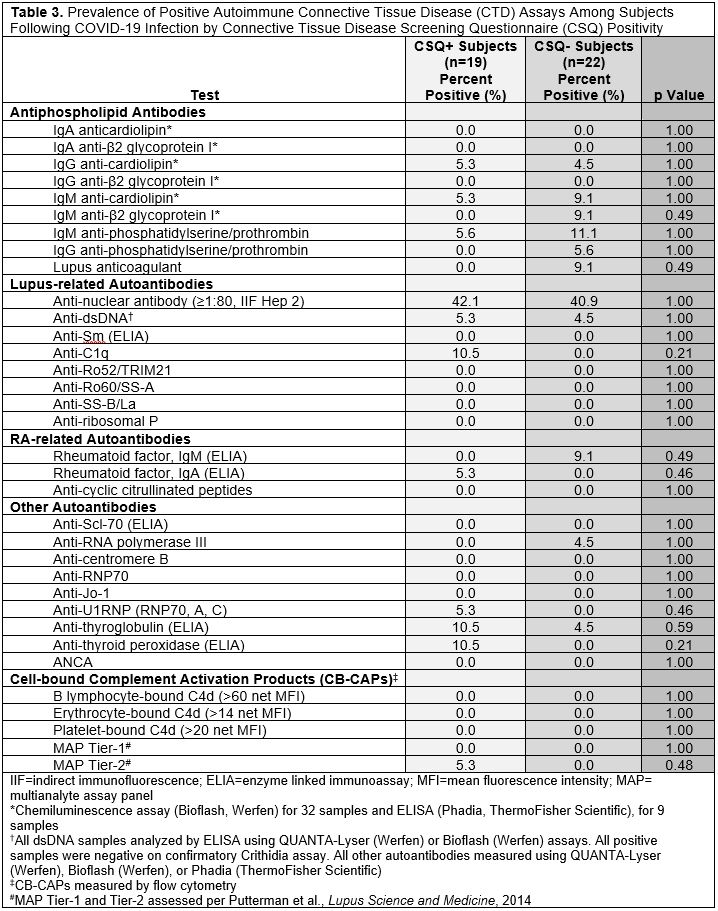 Table 3. Prevalence of Positive Autoimmune Connective Tissue Disease (CTD) Assays Among Subjects Following COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) Positivity
Table 3. Prevalence of Positive Autoimmune Connective Tissue Disease (CTD) Assays Among Subjects Following COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) Positivity
Disclosures: E. Oakes, None; J. Ellrodt, None; R. Alexander, Exagen Inc.; J. Conklin, Exagen, Inc.; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; H. Guan, None; S. Tedeschi, Moderna, NGM Biopharmaceuticals; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; S. Case, None; T. Hsu, None; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; A. Jonsson, Amgen; K. Costenbader, Eli Lilly, Janssen, Amgen, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline(GSK), Gilead, Exagen, Neutrolis, Cel-Sci, Alkermes.
Background/Purpose: Onset of autoimmune connective tissue disease (CTD) after COVID-19 infection has been reported and complement activation has been implicated. We examined common autoimmune rheumatic disease symptoms, serologies, and complement activation arising after COVID-19 infection.
Methods: We conducted a cross-sectional prospective study of patients who tested positive for COVID-19 from 3/1/20-9/1/21 at a large multihospital system. Eligible patients, ≥18 years old and ≥3 months post-COVID-19 infection, received the CTD Screening Questionnaire (CSQ; Karlson EW, 1995) and the Brief Pain Inventory (BPI) by electronic or physical mail. Subjects who returned completed questionnaires, had evidence of new CTD symptoms on CSQ, and had no prior CTD diagnosis (CSQ+) were invited for a physician exam and blood draw to test for autoimmune serologies and cell-bound complement activation products (EC4d, BC4d, and PC4d, together CB-CAPs). The multi-analyte assay panel (MAP) was determined using a two-tier algorithm. Targeting equal numbers of subjects who screened positive and negative on the CSQ, we invited age- and sex-matched subjects who had COVID-19, negative CSQs, and no prior CTD diagnosis (CSQ-) for blood collection. We tested for associations between CSQ positivity after COVID-19 and autoantibodies and CB-CAPs by Fisher exact test.
Results: We invited 101 eligible CSQ+ subjects and 122 CSQ- subjects to participate: 19 CSQ+ and 22 matched CSQ- subjects were enrolled. Age and sex were well-matched between the two groups (overall mean age 53.2 [SD 13.4], 82.9% female, 73.2% white). At time of infection, 4.9% of subjects were vaccinated and 14.6% were hospitalized (Table 1). Mean pain severity and interference scores were comparable among the CSQ+ and CSQ-. Among CSQ+ subjects, 11 subjects (57.9%) screened positive for symptoms of systemic lupus erythematosus (SLE), 8 (42.1%) screened positive for symptoms of rheumatoid arthritis (RA), and 2 (10.5%) screened positive for symptoms of scleroderma (SSc). Joint pain (36.8%), shortness of breath (36.8%), and other (non malar) rash (42.1%) were the most common subject-reported new onset CTD symptoms (Table 2). On exam, 4 cases of new joint pain and 4 cases of new non malar rash were found. There was an overall low prevalence of CTD autoantibodies and no positive CB-CAPs. No significant associations were identified between new CTD symptoms by CSQ and autoantibodies, CB-CAPs, or MAP (Table 3).
Conclusion: In this comparison of subjects following COVID-19 infection with or without self-reported new CTD symptoms, we found overall low prevalence of CTD symptoms on exam and CTD-related autoantibodies, and no associations between new CTD symptoms or physical exam findings and CTD autoantibodies, CB-CAPs, or MAP positivity. To our knowledge, this is the first study of CB-CAPs, a novel complement-based biomarker for SLE, in the context of COVID-19, which is thought to activate complement. These findings suggest that CSQ symptom positivity ≥3 months post-COVID-19 infection may not be associated with formation of CB-CAPs or CTD-associated autoantibodies.
 Table 1. Demographic and Clinical Information of Subjects Recovered From COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) Positivity
Table 1. Demographic and Clinical Information of Subjects Recovered From COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) Positivity  Table 2. Subject-Reported New-Onset Autoimmune Connective Tissue Disease (CTD) Symptoms Following COVID-19 Infection and Confirmation on Physician Exam (n=19)
Table 2. Subject-Reported New-Onset Autoimmune Connective Tissue Disease (CTD) Symptoms Following COVID-19 Infection and Confirmation on Physician Exam (n=19) Table 3. Prevalence of Positive Autoimmune Connective Tissue Disease (CTD) Assays Among Subjects Following COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) Positivity
Table 3. Prevalence of Positive Autoimmune Connective Tissue Disease (CTD) Assays Among Subjects Following COVID-19 Infection by Connective Tissue Disease Screening Questionnaire (CSQ) PositivityDisclosures: E. Oakes, None; J. Ellrodt, None; R. Alexander, Exagen Inc.; J. Conklin, Exagen, Inc.; M. Choi, AstraZeneca, MitogenDx, mallinckrodt, Janssen, AbbVie/Abbott, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol-Myers Squibb(BMS), Celltrion, Ferring, Fresenius Kabi, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; H. Guan, None; S. Tedeschi, Moderna, NGM Biopharmaceuticals; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; S. Case, None; T. Hsu, None; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; A. Jonsson, Amgen; K. Costenbader, Eli Lilly, Janssen, Amgen, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline(GSK), Gilead, Exagen, Neutrolis, Cel-Sci, Alkermes.

