Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1575: Characteristics of an Internet-Based, International Cohort of Patients with a Self-Reported Diagnosis of Urticarial Vasculitis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JS
Jason Springer, MD, MS
Vanderbilt University Medical Center
Franklin, TN, United States
Abstract Poster Presenter(s)
Jason Springer1, Tanaz Kermani2, Dianne Shaw3, Kalen Larson4, Cristina Burroughs5 and Peter Merkel6, 1Vanderbilt University Medical Center, Nashville, TN, 2University of California Los Angeles, Los Angeles, CA, 3Vasculitis Foundation, Chapel Hill, NC, 4Vasculitis Foundation, Kansas City, MO, 5University of South Florida, Tampa, FL, 6University of Pennsylvania, Philadelphia, PA
Background/Purpose: Urticarial vasculitis is a markedly rare disease, with an annual incidence of < 1 per million. Hypocomplementemia is associated with systemic features and a worse prognosis. The purpose of this study is to describe and validate this international cohort based on patient reported data.
Methods: Participant enrollment was via a prospective, international, internet-based longitudinal vasculitis registry. Participants with a self-reported diagnosis of urticarial vasculitis were included. Enrollment was from November 2014 to February 2022. On-line standardized forms included data on disease manifestations, diagnostic testing and treatment. Responses of "I don't know" or missing responses were excluded. The 2012 Chapel Hill Consensus Conference (CHCC) definition of urticarial vasculitis was felt met if patients reported a) biopsy showing vasculitis, and b) any of the following: kidney involvement, joint involvement, obstructive lung disease, or ocular involvement. Fisher exact test was used to make comparisons between groups.
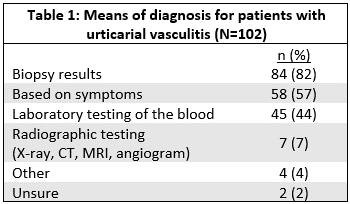
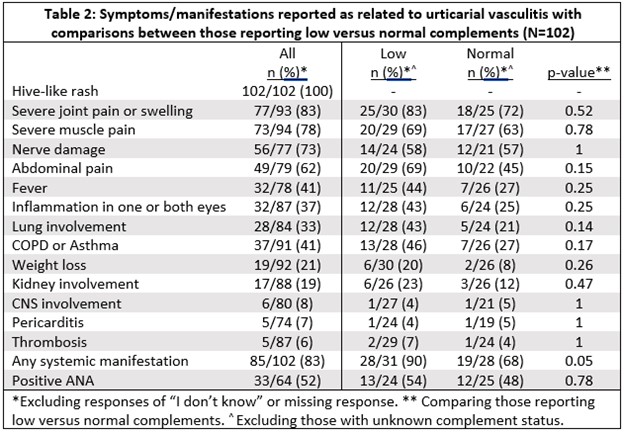
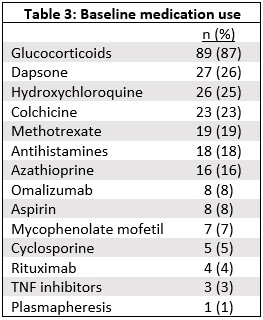
Results: Data from 102 participants with a self-reported diagnosis of urticarial vasculitis was used for this analysis. 92 (90%) were female. Mean age at onset of symptoms was 40.5 years old (SD 15.0) and at diagnosis was 44.1 years old (SD 13.2). All patients reported a hive-like rash. 90% (89/99) reported having a skin biopsy, of which 87% (77/89) reported the skin biopsy showed vasculitis. 84 patients (82%) reported that their diagnosis was based on the biopsy results (Table 1). 69% (37/54) reported a history of obstructive lung disease. Table 2 lists other reported manifestations/symptoms. 53% (31/59) report either a low C3 or C4. 74% (23/31) of those reporting low C3 or C4 met the CHCC definition for hypocomplementemic urticarial vasculitis. Systemic manifestations were more common in those reporting low C3 or C4 compared to those reporting normal C3 and C4 (90% vs 68%, p=0.05) (Table 2). 51% (34/67) report a positive anti-nuclear antibody. The physician specialty reported to manage the UV included: rheumatology 52 (51%), primary care 35 (34%), dermatologist 26 (25%), and Allergist/Immunologist 16 (16%). The medications most frequently reported used by patients are listed in Table 3.
Conclusion: The diagnosis of urticarial vasculitis is clinicopathological, based on both the clinical manifestation of urticaria and histologic confirmation of small-vessel vasculitis. All patients in this cohort reported urticarial rashes, the majority reported a skin biopsy showing vasculitis, and the manifestations of disease are consistent with physician-reported cohorts. This cohort has an overrepresentation of participants with more severe disease, possibly reflecting a higher propensity of such patients to participate in internet-based research. Internet-based cohorts, based on patient reported data, are means for the future conduct of large clinical trials in extremely rare diseases, such as urticarial vasculitis.
Disclosures: J. Springer, Chemocentryx; T. Kermani, None; D. Shaw, None; K. Larson, None; C. Burroughs, None; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate.
Background/Purpose: Urticarial vasculitis is a markedly rare disease, with an annual incidence of < 1 per million. Hypocomplementemia is associated with systemic features and a worse prognosis. The purpose of this study is to describe and validate this international cohort based on patient reported data.
Methods: Participant enrollment was via a prospective, international, internet-based longitudinal vasculitis registry. Participants with a self-reported diagnosis of urticarial vasculitis were included. Enrollment was from November 2014 to February 2022. On-line standardized forms included data on disease manifestations, diagnostic testing and treatment. Responses of "I don't know" or missing responses were excluded. The 2012 Chapel Hill Consensus Conference (CHCC) definition of urticarial vasculitis was felt met if patients reported a) biopsy showing vasculitis, and b) any of the following: kidney involvement, joint involvement, obstructive lung disease, or ocular involvement. Fisher exact test was used to make comparisons between groups.
Results: Data from 102 participants with a self-reported diagnosis of urticarial vasculitis was used for this analysis. 92 (90%) were female. Mean age at onset of symptoms was 40.5 years old (SD 15.0) and at diagnosis was 44.1 years old (SD 13.2). All patients reported a hive-like rash. 90% (89/99) reported having a skin biopsy, of which 87% (77/89) reported the skin biopsy showed vasculitis. 84 patients (82%) reported that their diagnosis was based on the biopsy results (Table 1). 69% (37/54) reported a history of obstructive lung disease. Table 2 lists other reported manifestations/symptoms. 53% (31/59) report either a low C3 or C4. 74% (23/31) of those reporting low C3 or C4 met the CHCC definition for hypocomplementemic urticarial vasculitis. Systemic manifestations were more common in those reporting low C3 or C4 compared to those reporting normal C3 and C4 (90% vs 68%, p=0.05) (Table 2). 51% (34/67) report a positive anti-nuclear antibody. The physician specialty reported to manage the UV included: rheumatology 52 (51%), primary care 35 (34%), dermatologist 26 (25%), and Allergist/Immunologist 16 (16%). The medications most frequently reported used by patients are listed in Table 3.
Conclusion: The diagnosis of urticarial vasculitis is clinicopathological, based on both the clinical manifestation of urticaria and histologic confirmation of small-vessel vasculitis. All patients in this cohort reported urticarial rashes, the majority reported a skin biopsy showing vasculitis, and the manifestations of disease are consistent with physician-reported cohorts. This cohort has an overrepresentation of participants with more severe disease, possibly reflecting a higher propensity of such patients to participate in internet-based research. Internet-based cohorts, based on patient reported data, are means for the future conduct of large clinical trials in extremely rare diseases, such as urticarial vasculitis.



Disclosures: J. Springer, Chemocentryx; T. Kermani, None; D. Shaw, None; K. Larson, None; C. Burroughs, None; P. Merkel, AbbVie, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Neutrolis, Takeda, CSL Behring, Dynacure, EMDSerono, Immagene, Jannsen, Kiniksa, Magenta, Novartis, Pfizer, Q32, Regeneron, Sparrow, Eicos, Electra, Kyverna, UpToDate.

