Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1491: Clinically Relevant Differences in the Mobility of Patients with Axial Spondyloarthritis Related to Daytime of Performance
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

David Kiefer, MD
Rheumazentrum Ruhrgebiet; Ruhr-Universität Bochum
Herne, Germany
Abstract Poster Presenter(s)
David Kiefer1, Juergen Braun1, Lucia Schneider1, Niklas Kolle1, Uta Kiltz1, Ioana Andreica2, Philipp Sewerin1, Bjoern Buehring3, susanne herbold1 and Xenofon Baraliakos4, 1Rheumazentrum Ruhrgebiet, Herne, Germany, 2Rheumazentrum Ruhrgebiet, Ruhr-Universität-Bochum, Herne, Germany, 3Bergisches Rheuma-Zentrum, Velbert, Germany, 4Rheumazentrum Ruhrgebiet Herne, Herne, Germany
Background/Purpose: Patients with axial spondyloarthritis (axSpA) often experience impairments of spinal mobility and physical function due to inflammation and structural damage. Back pain and morning stiffness are common symptoms and part of established disease activity criteria but are not included in mobility assessments. The diurnal rhythm of proinflammatory cytokines and cortisol is known to peak in the early morning hours. This study objective is to compare the performance of patients with axSpA regarding measures of mobility and physical function including objective electronic measurements with the Epionics SPINE device (ES) at different times of the day.
Methods: Consecutive inpatients with axSpA were examined once in the morning (V1: before 10:00am) and once in the afternoon (V2: after 02:00pm) by the Bath AS spinal mobility score (BASMI), the AS physical performance index (ASPI), the Short Physical Performance Battery (SPPB) and measurements with the ES, including range of motion (RoM) and kinematics (RoK). The results were compared using t-tests. Medication with NSAIDs and biologics was recorded.
Results: A total of 101 patients with axSpA (64 r-axSpA; 37 nr-axSpA) were included (Table 1). Significant differences between assessments performed in the morning vs. the afternoon were seen for ASPI, and SPPB, and for ES measures of speed (RoK; p< 0.018) but not for RoM, except for lateral flexion (p=0.002) (Table 2 and 3). Total BASMI scores slightly improved but single BASMI items showed variability (Table 2). Differences between nr-axSpA and r-axSpA were observed in age, sex, spinal mobility, CRP and percentage of HLA B27-positivity but not for disease activity and function (table 1).
Conclusion: Spinal mobility of patients with axSpA differs considerably at different times of the day, being worse in the morning and improving significantly in the afternoon. Importantly, this was most prominently shown by performance-based measures in comparison to standard assessments such as BASMI. Data on diurnal variation of mobility has important implications for clinical studies, strongly suggesting that the daytime of assessments needs to be standardized. Whether and how medication has an influence on this remains to be evaluated.
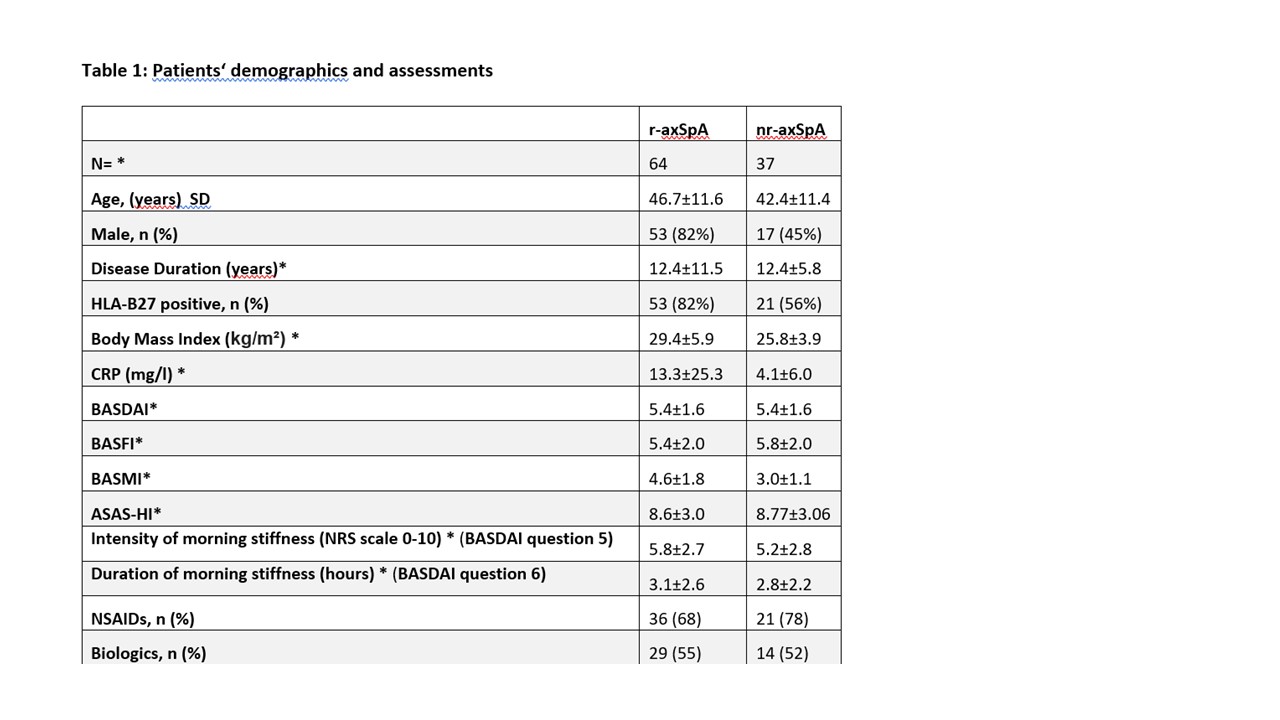 *variables are mean ± standard deviation if not otherwise indicated; BASMI: Bath Ankylosing Spondylitis (AS) Metrology Index; BASDAI: Bath AS Disease Activity Index; BASFI: Bath AS Functional Index; NSAIDs: Non-steroidal anti-inflammatory drugs; r-axSpA = radiographic axial spondyloarthritis (axSpA); non-radiographic axSpA (nr-axSpA); NRS = numerical rating scale
*variables are mean ± standard deviation if not otherwise indicated; BASMI: Bath Ankylosing Spondylitis (AS) Metrology Index; BASDAI: Bath AS Disease Activity Index; BASFI: Bath AS Functional Index; NSAIDs: Non-steroidal anti-inflammatory drugs; r-axSpA = radiographic axial spondyloarthritis (axSpA); non-radiographic axSpA (nr-axSpA); NRS = numerical rating scale
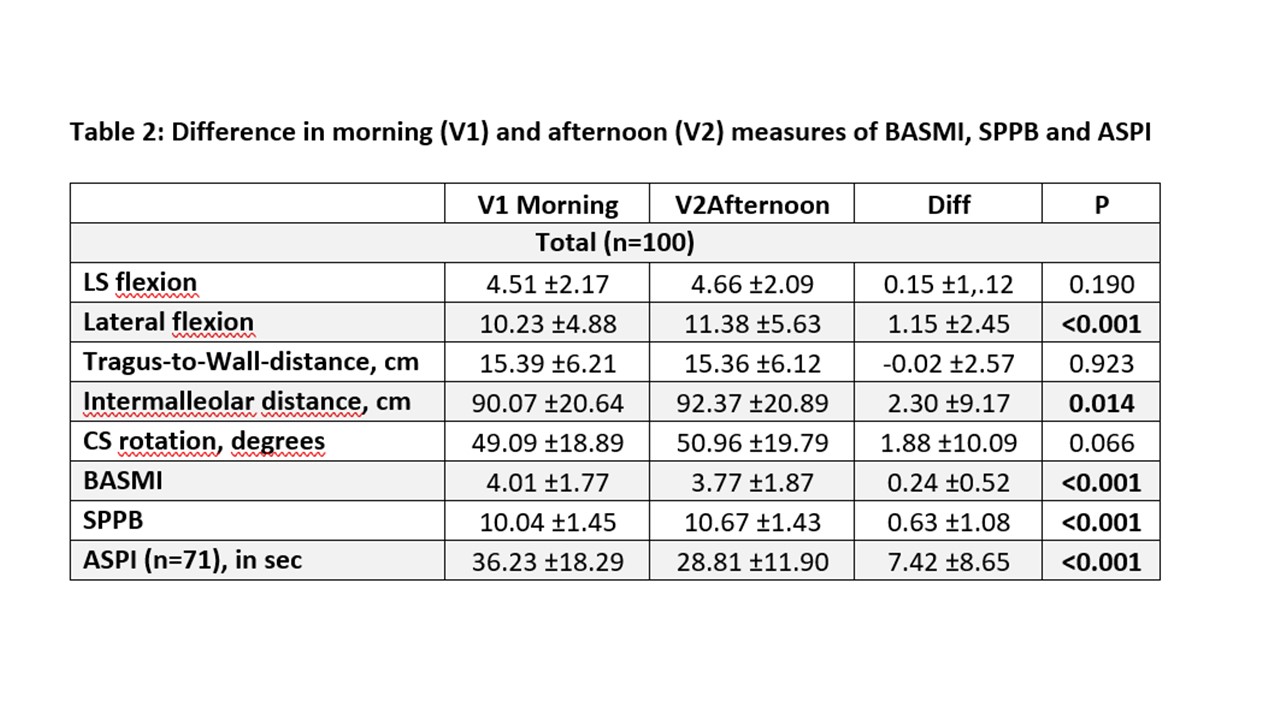 *variables are mean ± standard deviation if not otherwise indicated; LS: Lumbar spine, CS: cervical spine; BASMI: Bath Ankylosing Spondylitis Metrology Index, ASPI: Ankylosing Spondylitis Performance-based Improvement, SPPB: Short Physical Performance Battery
*variables are mean ± standard deviation if not otherwise indicated; LS: Lumbar spine, CS: cervical spine; BASMI: Bath Ankylosing Spondylitis Metrology Index, ASPI: Ankylosing Spondylitis Performance-based Improvement, SPPB: Short Physical Performance Battery
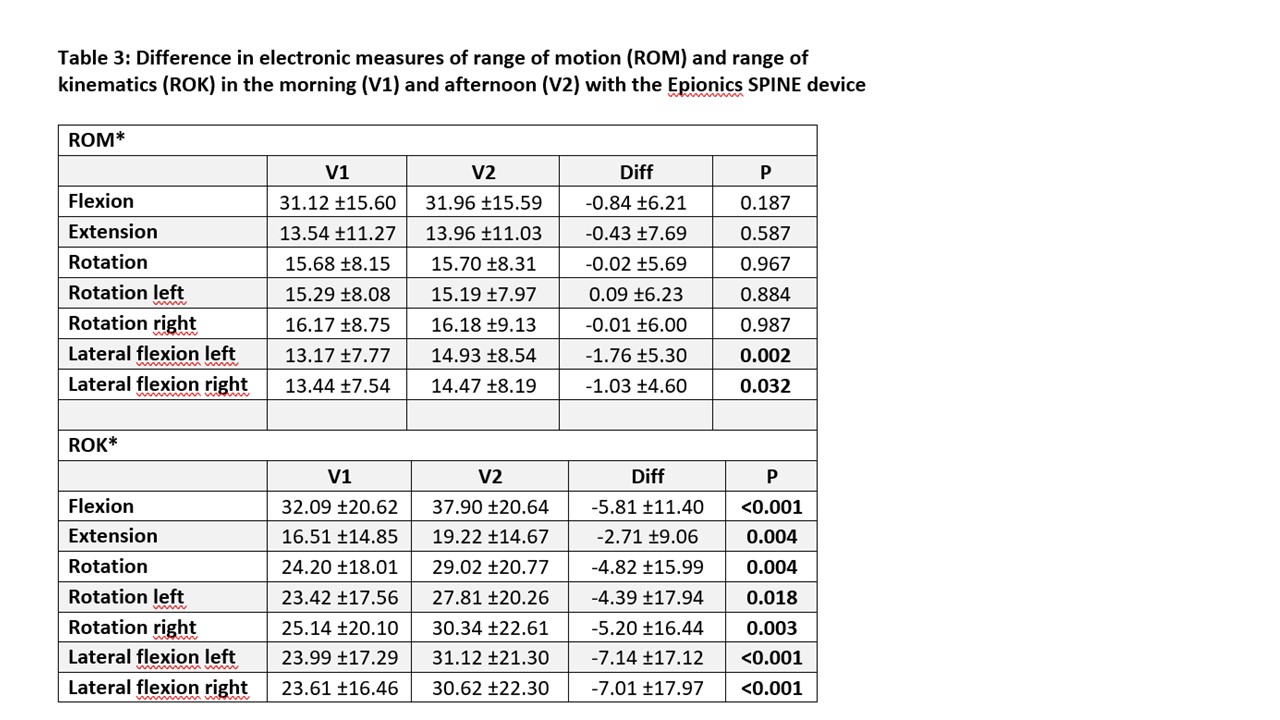 *variables are mean ± standard deviation if not otherwise indicated; Diff: Difference
*variables are mean ± standard deviation if not otherwise indicated; Diff: Difference
ROM: Range of motion in angular degrees/second;
ROK: range of kinematics in angular degrees/second;
Disclosures: D. Kiefer, Novartis, AbbVie/Abbott, Boehringer-Ingelheim, Janssen, UCB, Pfizer; J. Braun, None; L. Schneider, None; N. Kolle, None; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; I. Andreica, None; P. Sewerin, None; B. Buehring, AbbVie/Abbott, Gilead/Galapagos, Merck/MSD, Amgen, UCB, Biogen, Sanofi Genzyme, Theramex; s. herbold, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi.
Background/Purpose: Patients with axial spondyloarthritis (axSpA) often experience impairments of spinal mobility and physical function due to inflammation and structural damage. Back pain and morning stiffness are common symptoms and part of established disease activity criteria but are not included in mobility assessments. The diurnal rhythm of proinflammatory cytokines and cortisol is known to peak in the early morning hours. This study objective is to compare the performance of patients with axSpA regarding measures of mobility and physical function including objective electronic measurements with the Epionics SPINE device (ES) at different times of the day.
Methods: Consecutive inpatients with axSpA were examined once in the morning (V1: before 10:00am) and once in the afternoon (V2: after 02:00pm) by the Bath AS spinal mobility score (BASMI), the AS physical performance index (ASPI), the Short Physical Performance Battery (SPPB) and measurements with the ES, including range of motion (RoM) and kinematics (RoK). The results were compared using t-tests. Medication with NSAIDs and biologics was recorded.
Results: A total of 101 patients with axSpA (64 r-axSpA; 37 nr-axSpA) were included (Table 1). Significant differences between assessments performed in the morning vs. the afternoon were seen for ASPI, and SPPB, and for ES measures of speed (RoK; p< 0.018) but not for RoM, except for lateral flexion (p=0.002) (Table 2 and 3). Total BASMI scores slightly improved but single BASMI items showed variability (Table 2). Differences between nr-axSpA and r-axSpA were observed in age, sex, spinal mobility, CRP and percentage of HLA B27-positivity but not for disease activity and function (table 1).
Conclusion: Spinal mobility of patients with axSpA differs considerably at different times of the day, being worse in the morning and improving significantly in the afternoon. Importantly, this was most prominently shown by performance-based measures in comparison to standard assessments such as BASMI. Data on diurnal variation of mobility has important implications for clinical studies, strongly suggesting that the daytime of assessments needs to be standardized. Whether and how medication has an influence on this remains to be evaluated.
 *variables are mean ± standard deviation if not otherwise indicated; BASMI: Bath Ankylosing Spondylitis (AS) Metrology Index; BASDAI: Bath AS Disease Activity Index; BASFI: Bath AS Functional Index; NSAIDs: Non-steroidal anti-inflammatory drugs; r-axSpA = radiographic axial spondyloarthritis (axSpA); non-radiographic axSpA (nr-axSpA); NRS = numerical rating scale
*variables are mean ± standard deviation if not otherwise indicated; BASMI: Bath Ankylosing Spondylitis (AS) Metrology Index; BASDAI: Bath AS Disease Activity Index; BASFI: Bath AS Functional Index; NSAIDs: Non-steroidal anti-inflammatory drugs; r-axSpA = radiographic axial spondyloarthritis (axSpA); non-radiographic axSpA (nr-axSpA); NRS = numerical rating scale *variables are mean ± standard deviation if not otherwise indicated; LS: Lumbar spine, CS: cervical spine; BASMI: Bath Ankylosing Spondylitis Metrology Index, ASPI: Ankylosing Spondylitis Performance-based Improvement, SPPB: Short Physical Performance Battery
*variables are mean ± standard deviation if not otherwise indicated; LS: Lumbar spine, CS: cervical spine; BASMI: Bath Ankylosing Spondylitis Metrology Index, ASPI: Ankylosing Spondylitis Performance-based Improvement, SPPB: Short Physical Performance Battery *variables are mean ± standard deviation if not otherwise indicated; Diff: Difference
*variables are mean ± standard deviation if not otherwise indicated; Diff: DifferenceROM: Range of motion in angular degrees/second;
ROK: range of kinematics in angular degrees/second;
Disclosures: D. Kiefer, Novartis, AbbVie/Abbott, Boehringer-Ingelheim, Janssen, UCB, Pfizer; J. Braun, None; L. Schneider, None; N. Kolle, None; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; I. Andreica, None; P. Sewerin, None; B. Buehring, AbbVie/Abbott, Gilead/Galapagos, Merck/MSD, Amgen, UCB, Biogen, Sanofi Genzyme, Theramex; s. herbold, None; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi.

