Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1150–1165) Spondyloarthritis Including PsA – Basic Science Poster
1151: Colonic Organoids to Study the Role of HLA-B27 in Gastrointestinal Epithelial Biology
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
Tejpal Gill, PhD
OHSU
Portland, OR, United States
Abstract Poster Presenter(s)
Alec Furst1, John Davis1, Manuel Rodriguez1, Kimberly Ogle1, James T. Rosenbaum2 and Tejpal Gill1, 1OHSU, Portland, OR, 2Legacy Devers Eye Institute, Portland, OR
Background/Purpose: Axial spondyloarthritis (axSpA) is defined clinically as inflammatory back pain along with peripheral arthritis, and extra-articular manifestations involving the eye, gut, and skin. Even though the exact mechanisms underlying HLA-B27 associated axSpA and disease susceptibility are unknown, several observations including microscopic gut lesions, clinical overlap with Crohn's disease, and microbial dysbiosis point to the gut as a potential primary cause. However, the role of HLA-B27 associated alteration in gut epithelial biology or host-microbe interaction is not clear in axSpA pathogenesis. Therefore, we have developed colonic organoids from HLA-B27 positive and negative healthy individuals, axSpA and IBD patients, as well as HLA-B27 transgenic (Tg) and wild-type (Wt) rats to study the effect of HLA-B27 on host-microbe interactions and epithelial cell biology.
Methods: To generate human colonic organoids, frozen biopsies from healthy individuals undergoing elective colonoscopies, and axSpA and IBD patients undergoing scheduled colonoscopies were used. These biopsies were minced and the tissue was digested on a shaker to release the crypts. The crypts were washed and resuspended in undiluted Cultrex reduced-growth-factor basement membrane extract. The organoids were expanded after 4-7 days by mechanical disruption and splitting. Rat organoids from HLA-B27 Tg and Wt animals were generated from colon tissue using a method similar to human organoid generation with sight modifications. The organoids were stained with antibodies for various epithelial markers for cell differentiation (Ki67), tight junction proteins (Occludin, Claudin-4,) and goblet cells (Muc2) and compared among healthy individuals with and without HLA-B27, HLA-B27 positive axSpA patients, and IBD patients.
Results: We developed colonic organoids from multiple HLA-B27 positive and negative individuals (Figure 1) and demonstrated that organoids maintain their stemness and ability to differentiate into mature (lobular) organoids and epithelial monolayers. Immunofluorescence studies revealed a decreased mucin production in goblet cells (Muc2), while we did not observe any changes in cell proliferation (Ki67) or tight junction proteins (Occludin, Claudin-4) in HLA-B27 positive healthy individuals, as well as axSpA and IBD patients in comparison with the HLA-B27 negative healthy individuals. Since our sample numbers are low (N=1-3/group), we are confirming these results on more organoid samples. Immunohistochemistry analysis has also shown less mucin production in the goblet cells from the colon of HLA-B27 Tg rats. To determine host-microbe interactions at the epithelial surface, we are characterizing gene dysregulation in endotoxin-treated colonic organoids using RNA-seq analysis.
Conclusion: The preliminary results reveal decreased mucus production in HLA-B27 positive healthy individuals and patients with axSpA and IBD. Furthermore, mechanistic studies to determine the effect of HLA-B27 on decreased mucus production are underway.
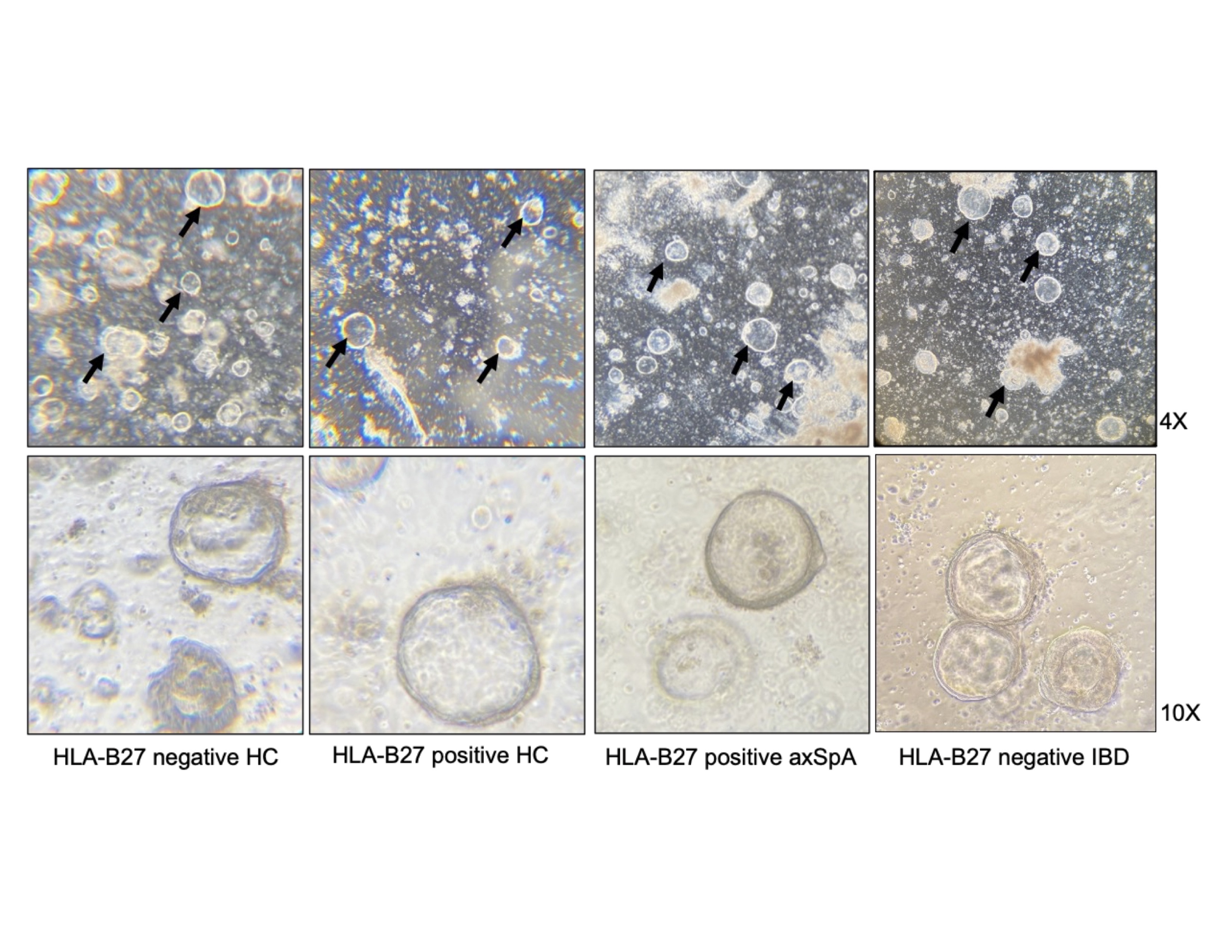 Representative images from HLA-B27 negative healthy control (HC), HLA-B27 positive HC, HLA-B27 positive axSpA, and HLA-B27 negative IBD patient colonic organoid lines. Organoids are shown after the establishment of cultures (day 4), at 4x (top panel) or 10x (bottom panel). The arrows are pointing to spheroids or early-stage organoids.
Representative images from HLA-B27 negative healthy control (HC), HLA-B27 positive HC, HLA-B27 positive axSpA, and HLA-B27 negative IBD patient colonic organoid lines. Organoids are shown after the establishment of cultures (day 4), at 4x (top panel) or 10x (bottom panel). The arrows are pointing to spheroids or early-stage organoids.
Disclosures: A. Furst, None; J. Davis, None; M. Rodriguez, None; K. Ogle, None; J. Rosenbaum, Abbvie, Gilead, Novartis, Horizon Therapeutics, Eli Lilly, Celgene, Pfizer, UpToDate, Revolo, Priovant, Corvus, Affibody; T. Gill, None.
Background/Purpose: Axial spondyloarthritis (axSpA) is defined clinically as inflammatory back pain along with peripheral arthritis, and extra-articular manifestations involving the eye, gut, and skin. Even though the exact mechanisms underlying HLA-B27 associated axSpA and disease susceptibility are unknown, several observations including microscopic gut lesions, clinical overlap with Crohn's disease, and microbial dysbiosis point to the gut as a potential primary cause. However, the role of HLA-B27 associated alteration in gut epithelial biology or host-microbe interaction is not clear in axSpA pathogenesis. Therefore, we have developed colonic organoids from HLA-B27 positive and negative healthy individuals, axSpA and IBD patients, as well as HLA-B27 transgenic (Tg) and wild-type (Wt) rats to study the effect of HLA-B27 on host-microbe interactions and epithelial cell biology.
Methods: To generate human colonic organoids, frozen biopsies from healthy individuals undergoing elective colonoscopies, and axSpA and IBD patients undergoing scheduled colonoscopies were used. These biopsies were minced and the tissue was digested on a shaker to release the crypts. The crypts were washed and resuspended in undiluted Cultrex reduced-growth-factor basement membrane extract. The organoids were expanded after 4-7 days by mechanical disruption and splitting. Rat organoids from HLA-B27 Tg and Wt animals were generated from colon tissue using a method similar to human organoid generation with sight modifications. The organoids were stained with antibodies for various epithelial markers for cell differentiation (Ki67), tight junction proteins (Occludin, Claudin-4,) and goblet cells (Muc2) and compared among healthy individuals with and without HLA-B27, HLA-B27 positive axSpA patients, and IBD patients.
Results: We developed colonic organoids from multiple HLA-B27 positive and negative individuals (Figure 1) and demonstrated that organoids maintain their stemness and ability to differentiate into mature (lobular) organoids and epithelial monolayers. Immunofluorescence studies revealed a decreased mucin production in goblet cells (Muc2), while we did not observe any changes in cell proliferation (Ki67) or tight junction proteins (Occludin, Claudin-4) in HLA-B27 positive healthy individuals, as well as axSpA and IBD patients in comparison with the HLA-B27 negative healthy individuals. Since our sample numbers are low (N=1-3/group), we are confirming these results on more organoid samples. Immunohistochemistry analysis has also shown less mucin production in the goblet cells from the colon of HLA-B27 Tg rats. To determine host-microbe interactions at the epithelial surface, we are characterizing gene dysregulation in endotoxin-treated colonic organoids using RNA-seq analysis.
Conclusion: The preliminary results reveal decreased mucus production in HLA-B27 positive healthy individuals and patients with axSpA and IBD. Furthermore, mechanistic studies to determine the effect of HLA-B27 on decreased mucus production are underway.
 Representative images from HLA-B27 negative healthy control (HC), HLA-B27 positive HC, HLA-B27 positive axSpA, and HLA-B27 negative IBD patient colonic organoid lines. Organoids are shown after the establishment of cultures (day 4), at 4x (top panel) or 10x (bottom panel). The arrows are pointing to spheroids or early-stage organoids.
Representative images from HLA-B27 negative healthy control (HC), HLA-B27 positive HC, HLA-B27 positive axSpA, and HLA-B27 negative IBD patient colonic organoid lines. Organoids are shown after the establishment of cultures (day 4), at 4x (top panel) or 10x (bottom panel). The arrows are pointing to spheroids or early-stage organoids.Disclosures: A. Furst, None; J. Davis, None; M. Rodriguez, None; K. Ogle, None; J. Rosenbaum, Abbvie, Gilead, Novartis, Horizon Therapeutics, Eli Lilly, Celgene, Pfizer, UpToDate, Revolo, Priovant, Corvus, Affibody; T. Gill, None.

