Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1186–1214) Epidemiology and Public Health Poster II
1205: Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines Against COVID-19 Infection Among Patients with Systemic Autoimmune Rheumatic Diseases
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- ZW
Zachary Wallace, MD, MSc
Massachusetts General Hospital
Newton, MA, United States
Abstract Poster Presenter(s)
Claire Cook1, Naomi Patel2, Xiaoqing Fu1, Xiaosong Wang3, Yumeko Kawano3, Kathleen Vanni3, Grace Qian3, Emily Banasiak3, Emily Kowalski3, Hyon Choi4, yuqing zhang5, Jeffrey Sparks6 and Zachary Wallace1, 1Massachusetts General Hospital, Boston, MA, 2Massachusetts General Hospital, Sale Creek, TN, 3Brigham and Women's Hospital, Boston, MA, 4MASSACHUSETTS GENERAL HOSPITAL, Lexington, MA, 5Massachusetts General Hospital, Quincy, MA, 6Brigham and Women's Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Patients with systemic autoimmune rheumatic diseases (SARDs) on immunomodulatory medications may be at particular risk for COVID-19 breakthrough infection given uncertain immune response to COVID-19 vaccination. We aimed to assess the comparative effectiveness of BNT162b2 and mRNA-1273 among patients with SARDs against COVID-19 infection.
Methods: In this retrospective cohort study, we identified patients with SARDs being treated with DMARDs and/or glucocorticoids in the Massachusetts (MA) system who received at least two doses of either the BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccines between 12/27/2020 and 5/15/2021. Patients who received other or mixed vaccine doses as part of their initial two dose series were excluded. Patients were followed from index date (date of first vaccine dose) until the date of positive SARS-CoV-2 test result (PCR or antigen), death, or end of follow-up (2/22/2022).To account for potential confounding introduced by variation in vaccine administration and availability, and fluctuation of COVID-19 infection rates over time, we divided the study period into two-week time blocks and calculated the time-block specific propensity score for the BNT162b2 vaccine using the logistic regression model that included potential confounders. We compared the risk of COVID-19 breakthrough infection between BNT162b2 and mRNA-1273 vaccine recipients using overlap propensity score–weighted cox proportional hazard models.
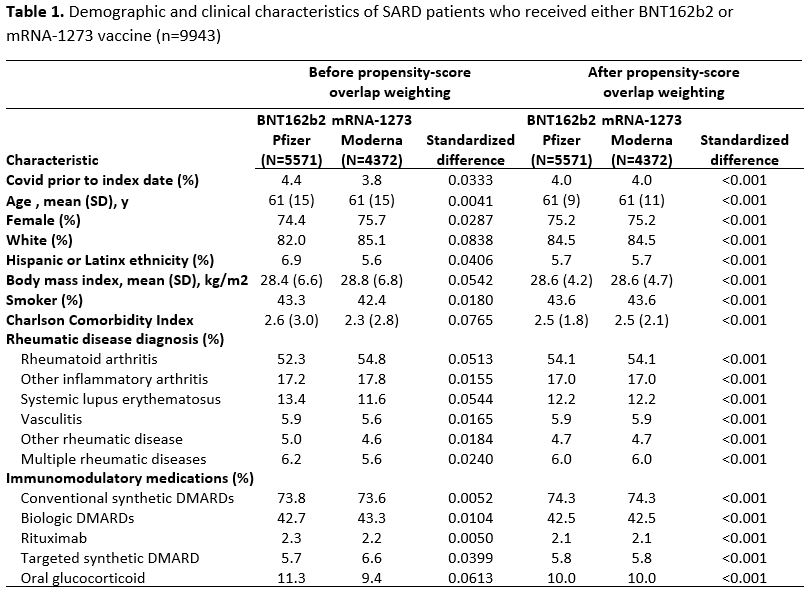
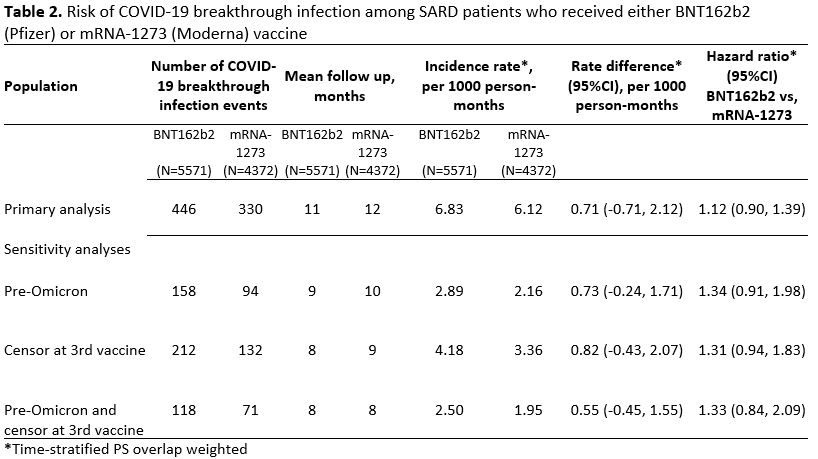
Results: We identified 9943 SARD patients who received either BNT162b2 or mRNA-1273. Demographics and clinical characteristics were similar in both groups prior to weighting: mean age 61 vs. 61 years, female 74% vs 76%, White 82% vs. 85%, most frequent SARD (rheumatoid arthritis, 52% vs. 55%) and treatment (conventional DMARD, 74% vs. 74%) (Table 1). Of 5571 patients who received BNT162b2, 446 had a breakthrough infection (IR 6.83 per 1000 person-months), vs 330 among 4372 mRNA-1273 recipients (IR 6.12 per 1000 person-months) (Table 2). The time-stratified PS weighted rate difference of breakthrough COVID-19 infection for BNT162b2 vs. mRNA-1273 was 0.71 per 1000 person months (95%CI: -0.71, 2.12) with a corresponding weighted hazard ratio of 1.12 (95%CI: 0.90, 1.39) (Figure 1). When patients were censored at the time of a 3rd vaccine or follow-up ended prior to Omicron dominance in MA, there was a trend towards higher breakthrough infection risk with BNT162b2 vs mRNA-1273, though this was not statistically significant (Table 2).
Conclusion: Among SARDs on immunomodulators, the risk of breakthrough COVID-19 infection is low after receiving either BNT162b2 or mRNA-1273. However, there was a trend toward greater benefit with mRNA-1273 vs BNT162b2, especially prior to a 3rd vaccine dose and the Omicron wave. These results are similar to previous observations made in the general population1. Ongoing real-world studies are needed to investigate the risk of breakthrough infection among SARDs as vaccination and mitigation strategies, infection rates, and new variants evolve.
1Dickerman et al., N Engl J Med, 2022;386(2):105-115.
.jpg)
Disclosures: C. Cook, None; N. Patel, FVC Health; X. Fu, None; X. Wang, None; Y. Kawano, None; K. Vanni, None; G. Qian, None; E. Banasiak, None; E. Kowalski, None; H. Choi, Horizon, Allena, LG, Protalix; y. zhang, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon.
Background/Purpose: Patients with systemic autoimmune rheumatic diseases (SARDs) on immunomodulatory medications may be at particular risk for COVID-19 breakthrough infection given uncertain immune response to COVID-19 vaccination. We aimed to assess the comparative effectiveness of BNT162b2 and mRNA-1273 among patients with SARDs against COVID-19 infection.
Methods: In this retrospective cohort study, we identified patients with SARDs being treated with DMARDs and/or glucocorticoids in the Massachusetts (MA) system who received at least two doses of either the BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccines between 12/27/2020 and 5/15/2021. Patients who received other or mixed vaccine doses as part of their initial two dose series were excluded. Patients were followed from index date (date of first vaccine dose) until the date of positive SARS-CoV-2 test result (PCR or antigen), death, or end of follow-up (2/22/2022).To account for potential confounding introduced by variation in vaccine administration and availability, and fluctuation of COVID-19 infection rates over time, we divided the study period into two-week time blocks and calculated the time-block specific propensity score for the BNT162b2 vaccine using the logistic regression model that included potential confounders. We compared the risk of COVID-19 breakthrough infection between BNT162b2 and mRNA-1273 vaccine recipients using overlap propensity score–weighted cox proportional hazard models.
Results: We identified 9943 SARD patients who received either BNT162b2 or mRNA-1273. Demographics and clinical characteristics were similar in both groups prior to weighting: mean age 61 vs. 61 years, female 74% vs 76%, White 82% vs. 85%, most frequent SARD (rheumatoid arthritis, 52% vs. 55%) and treatment (conventional DMARD, 74% vs. 74%) (Table 1). Of 5571 patients who received BNT162b2, 446 had a breakthrough infection (IR 6.83 per 1000 person-months), vs 330 among 4372 mRNA-1273 recipients (IR 6.12 per 1000 person-months) (Table 2). The time-stratified PS weighted rate difference of breakthrough COVID-19 infection for BNT162b2 vs. mRNA-1273 was 0.71 per 1000 person months (95%CI: -0.71, 2.12) with a corresponding weighted hazard ratio of 1.12 (95%CI: 0.90, 1.39) (Figure 1). When patients were censored at the time of a 3rd vaccine or follow-up ended prior to Omicron dominance in MA, there was a trend towards higher breakthrough infection risk with BNT162b2 vs mRNA-1273, though this was not statistically significant (Table 2).
Conclusion: Among SARDs on immunomodulators, the risk of breakthrough COVID-19 infection is low after receiving either BNT162b2 or mRNA-1273. However, there was a trend toward greater benefit with mRNA-1273 vs BNT162b2, especially prior to a 3rd vaccine dose and the Omicron wave. These results are similar to previous observations made in the general population1. Ongoing real-world studies are needed to investigate the risk of breakthrough infection among SARDs as vaccination and mitigation strategies, infection rates, and new variants evolve.
1Dickerman et al., N Engl J Med, 2022;386(2):105-115.


.jpg)
Disclosures: C. Cook, None; N. Patel, FVC Health; X. Fu, None; X. Wang, None; Y. Kawano, None; K. Vanni, None; G. Qian, None; E. Banasiak, None; E. Kowalski, None; H. Choi, Horizon, Allena, LG, Protalix; y. zhang, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; Z. Wallace, Sanofi, Bristol-Myers Squibb(BMS), Zenas Biopharma, Shionogi, Horizon.

