Back
Abstract Session
Crystal arthropathies
Session: Abstracts: Metabolic and Crystal Arthropathies – Basic and Clinical Science (1579–1584)
1582: Allopurinol Use and Risk of Acute Coronary Syndrome in Patients with Incident Gout: A Population-based Study in Sweden
Sunday, November 13, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 114 Nutter Theatre
- PD
Panagiota Drivelegka, MD, PhD
Department of Rheumatology, Sahlgrenska University hospital
Gothenburg, Sweden
Presenting Author(s)
Panagiota Drivelegka1, Lennart Jacobsson2, Karin Bengtsson3 and Mats Dehlin4, 1Department of Rheumatology, Sahlgrenska University Hospital, Gothenburg, Sweden, 2Department of Rheumatology and Inflammation Research, Sahlgrenska Academy at Gothenburg University, Gothenburg, Sweden, 3Department of Rheumatology and Inflammation research, University of Gothenburg, Salhgrenska Academy, Gothenburg, Sweden, 4Dept of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Background/Purpose: Gout is associated with an increased risk of cardiovascular disease, with accumulated evidence that gout is an independent risk factor. Whether allopurinol use alters this risk is not clear. The aim of this study was to investigate the effect of allopurinol use on risk of first-ever acute coronary syndrome (ACS) in patients with incident gout.
Methods: Using data from regional and national population-based registers, we identified all patients in Western Sweden with first ICD-coded diagnosis for gout (ICD10: M10, M14.0, M14.1) in the period 2007 ̶ 2017 and without any previous allopurinol exposure (N=19,054; 67% males). Patients with a prior history of coronary heart disease were excluded. The outcome of interest was the occurrence of first-ever ACS event (ICD10: I20.0, I21) in the compulsory health care registers. The follow-up began from the first ICD-coded diagnosis for gout and ended at the earliest of: the outcome, death, emigration, or the end of study on 31 December 2017. Exposure to allopurinol at the end of follow-up was defined as: none (no dispensed prescription within 125 days from the end of follow-up) (reference group), 100mg, or >100mg, according to the last dispensed prescription within 125 days from the end of follow-up. Odds ratios (OR) and 95% confidence intervals (CI) for first-ever ACS event were calculated using logistic regression models with adjustments for age, sex, education level, comorbidity index (based on the number of ever diagnosed comorbidities, see included comorbidities in footnote of Table 1), dispensed prescriptions for cardiovascular drugs, anticoagulants/ platelet aggregation inhibitors, and/or cortisone within six months from the end of follow-up), and follow-up time.
Results: Women were older than men in all exposure categories (Table 1). Exposure to allopurinol was associated with older age, longer follow-up time and more comorbidities (Table 1). Exposure to 100mg and >100mg allopurinol had significantly lower OR for first-ever ACS event, as compared to those not exposed, overall (OR, 0.72; 95%CI, 0.59-0.88; and OR, 0.54; 95%CI, 0.40-0.72, respectively) and in men (OR, 0.72; 95%CI, 0.57-0.92; and OR, 0.57; 95%CI, 0.40-0.79, respectively). In women, exposure to > 100mg was associated with significantly lower OR for first-ever ACS event (OR, 0.46; 95%CI, 0.25-0.85), whereas exposure to 100mg was not (OR, 0.72; 95%CI, 0.50-1.02). Exposure to >100mg allopurinol was associated with lower OR for first-ever ACS event than exposure to 100mg in both men and women (Figure 1). The OR for first-ever ACS event were similar in men and women.
Conclusion: Current allopurinol use was associated with significantly lower risk of first-ever ACS event in patients with incident gout in a dose-dependent fashion, suggesting a protective effect for allopurinol. The effect of allopurinol was similar in men and women.
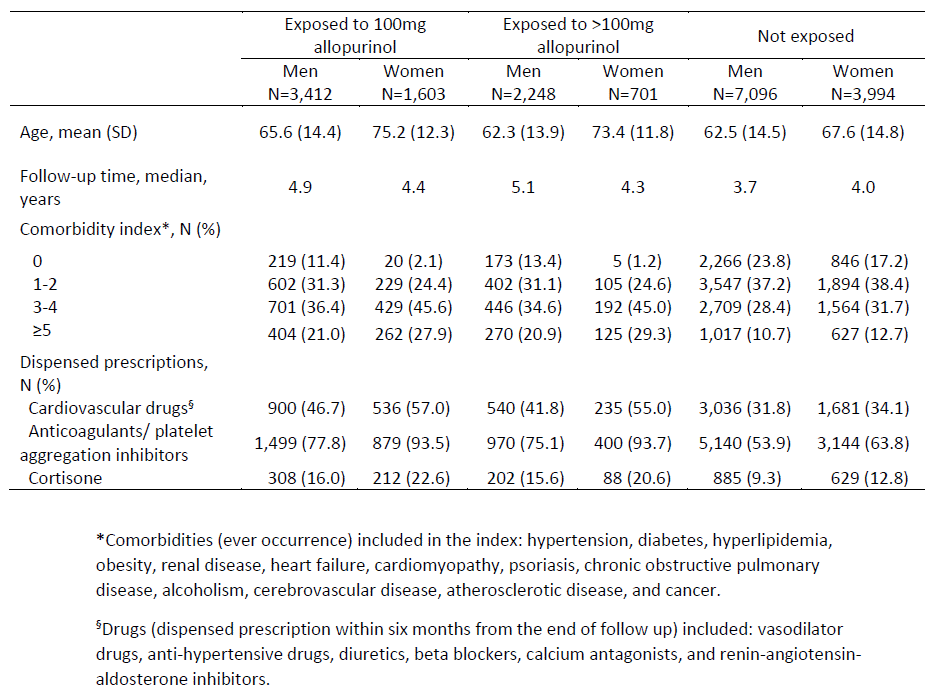 Table 1. Demographic characteristics of patients with incident gout, comorbidity index, and dispensed prescriptions, stratified by allopurinol exposure at the end of follow-up, and sex.
Table 1. Demographic characteristics of patients with incident gout, comorbidity index, and dispensed prescriptions, stratified by allopurinol exposure at the end of follow-up, and sex.
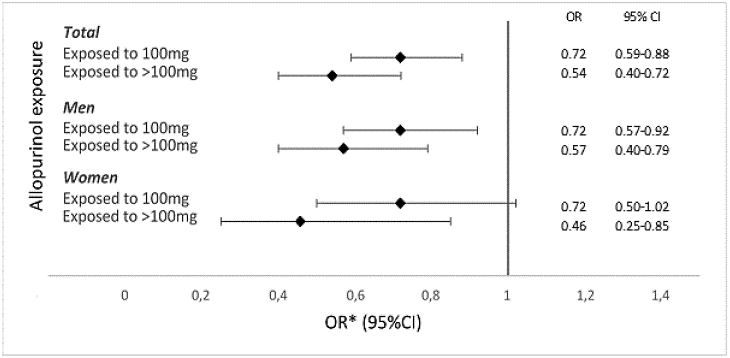 Figure 1. Adjusted odds ratios (OR) and 95% confidence intervals (CI) for first-ever acute coronary syndrome event in patients with incident gout, stratified by allopurinol exposure at the end of follow-up, and sex.
Figure 1. Adjusted odds ratios (OR) and 95% confidence intervals (CI) for first-ever acute coronary syndrome event in patients with incident gout, stratified by allopurinol exposure at the end of follow-up, and sex.
*Adjusted for age, sex, education level, comorbidity index, dispensed prescriptions for cardiovascular drugs, anticoagulants/ platelet aggregation inhibitors, and/or cortisone, and follow-up time.
Disclosures: P. Drivelegka, None; L. Jacobsson, Novartis, Eli Lilly, Janssen; K. Bengtsson, None; M. Dehlin, None.
Background/Purpose: Gout is associated with an increased risk of cardiovascular disease, with accumulated evidence that gout is an independent risk factor. Whether allopurinol use alters this risk is not clear. The aim of this study was to investigate the effect of allopurinol use on risk of first-ever acute coronary syndrome (ACS) in patients with incident gout.
Methods: Using data from regional and national population-based registers, we identified all patients in Western Sweden with first ICD-coded diagnosis for gout (ICD10: M10, M14.0, M14.1) in the period 2007 ̶ 2017 and without any previous allopurinol exposure (N=19,054; 67% males). Patients with a prior history of coronary heart disease were excluded. The outcome of interest was the occurrence of first-ever ACS event (ICD10: I20.0, I21) in the compulsory health care registers. The follow-up began from the first ICD-coded diagnosis for gout and ended at the earliest of: the outcome, death, emigration, or the end of study on 31 December 2017. Exposure to allopurinol at the end of follow-up was defined as: none (no dispensed prescription within 125 days from the end of follow-up) (reference group), 100mg, or >100mg, according to the last dispensed prescription within 125 days from the end of follow-up. Odds ratios (OR) and 95% confidence intervals (CI) for first-ever ACS event were calculated using logistic regression models with adjustments for age, sex, education level, comorbidity index (based on the number of ever diagnosed comorbidities, see included comorbidities in footnote of Table 1), dispensed prescriptions for cardiovascular drugs, anticoagulants/ platelet aggregation inhibitors, and/or cortisone within six months from the end of follow-up), and follow-up time.
Results: Women were older than men in all exposure categories (Table 1). Exposure to allopurinol was associated with older age, longer follow-up time and more comorbidities (Table 1). Exposure to 100mg and >100mg allopurinol had significantly lower OR for first-ever ACS event, as compared to those not exposed, overall (OR, 0.72; 95%CI, 0.59-0.88; and OR, 0.54; 95%CI, 0.40-0.72, respectively) and in men (OR, 0.72; 95%CI, 0.57-0.92; and OR, 0.57; 95%CI, 0.40-0.79, respectively). In women, exposure to > 100mg was associated with significantly lower OR for first-ever ACS event (OR, 0.46; 95%CI, 0.25-0.85), whereas exposure to 100mg was not (OR, 0.72; 95%CI, 0.50-1.02). Exposure to >100mg allopurinol was associated with lower OR for first-ever ACS event than exposure to 100mg in both men and women (Figure 1). The OR for first-ever ACS event were similar in men and women.
Conclusion: Current allopurinol use was associated with significantly lower risk of first-ever ACS event in patients with incident gout in a dose-dependent fashion, suggesting a protective effect for allopurinol. The effect of allopurinol was similar in men and women.
 Table 1. Demographic characteristics of patients with incident gout, comorbidity index, and dispensed prescriptions, stratified by allopurinol exposure at the end of follow-up, and sex.
Table 1. Demographic characteristics of patients with incident gout, comorbidity index, and dispensed prescriptions, stratified by allopurinol exposure at the end of follow-up, and sex. Figure 1. Adjusted odds ratios (OR) and 95% confidence intervals (CI) for first-ever acute coronary syndrome event in patients with incident gout, stratified by allopurinol exposure at the end of follow-up, and sex.
Figure 1. Adjusted odds ratios (OR) and 95% confidence intervals (CI) for first-ever acute coronary syndrome event in patients with incident gout, stratified by allopurinol exposure at the end of follow-up, and sex. *Adjusted for age, sex, education level, comorbidity index, dispensed prescriptions for cardiovascular drugs, anticoagulants/ platelet aggregation inhibitors, and/or cortisone, and follow-up time.
Disclosures: P. Drivelegka, None; L. Jacobsson, Novartis, Eli Lilly, Janssen; K. Bengtsson, None; M. Dehlin, None.

