Back
Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including PsA – Treatment II: PsA (1597–1602)
1600: The Impact of Second-Line Therapeutic on Disease Control After Discontinuation of First Line TNF Inhibitor in Patients with PsA: Analysis from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry
Sunday, November 13, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 201
.png)
Alexis Ogdie, MD
Rheumatologist, Associate Professor of Medicine
University of Pennsylvania
Philadelphia, PA, United States
Presenting Author(s)
Alexis Ogdie1, Robert McLean2, Taylor Blachley2, Nicole Middaugh2, Manish Mittal3, Jerry Clewell3, Sandra Ciecinski4 and Philip J Mease5, 1Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, 2CorEvitas, LLC, Waltham, MA, 3AbbVie, Inc., North Chicago, IL, 4AbbVie, Inc., Mettawa, IL, 5Swedish Medical Center/Providence St. Joseph Health, Seattle, WA
Background/Purpose: While evidence in patients (pts) with RA suggests that switching to a therapy with a different mechanism of action (MOA) may be more effective than cycling among TNF inhibitors (TNFi) after discontinuing a 1st line TNFi, the relative effectiveness of cycling vs switching in pts with PsA is currently unknown. This study compared clinical outcomes between pts with PsA who initiated a TNFi vs a non-TNFi biologic after discontinuing a 1st line TNFi in a real-world setting.
Methods: Pts with a clinical diagnosis of PsA enrolled in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry who initiated a 2nd line advanced therapy (baseline) after discontinuation of 1st line TNFi between May 2013–January 2022 were included. Eligible pts had a follow-up visit 6-months after initiating the 2nd line therapy. Pts were stratified into two cohorts: 1) those who initiated a 2nd line TNFi (cyclers) and 2) those who initiated a 2nd line non-TNFi biologic (switchers). Baseline characteristics were summarized descriptively and compared between the groups using standardized differences (d; 0.2, 0.5, 0.8 considered small, medium, and large effects). Multivariable adjusted Poisson regression models were used to calculate risk ratios (RR) and 95% confidence intervals (CI) estimating the association between cycling vs switching status and clinical outcomes at 6 months.
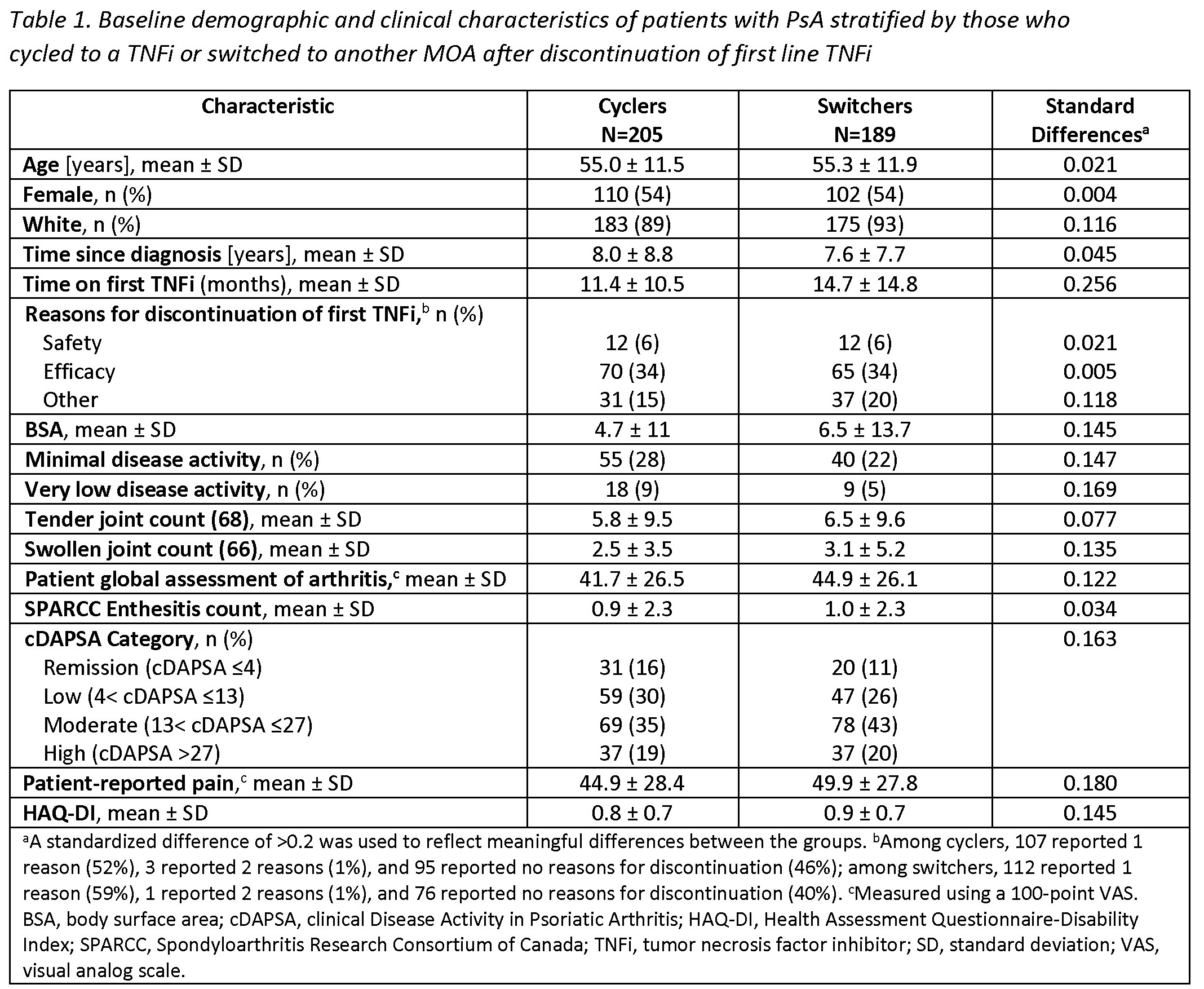
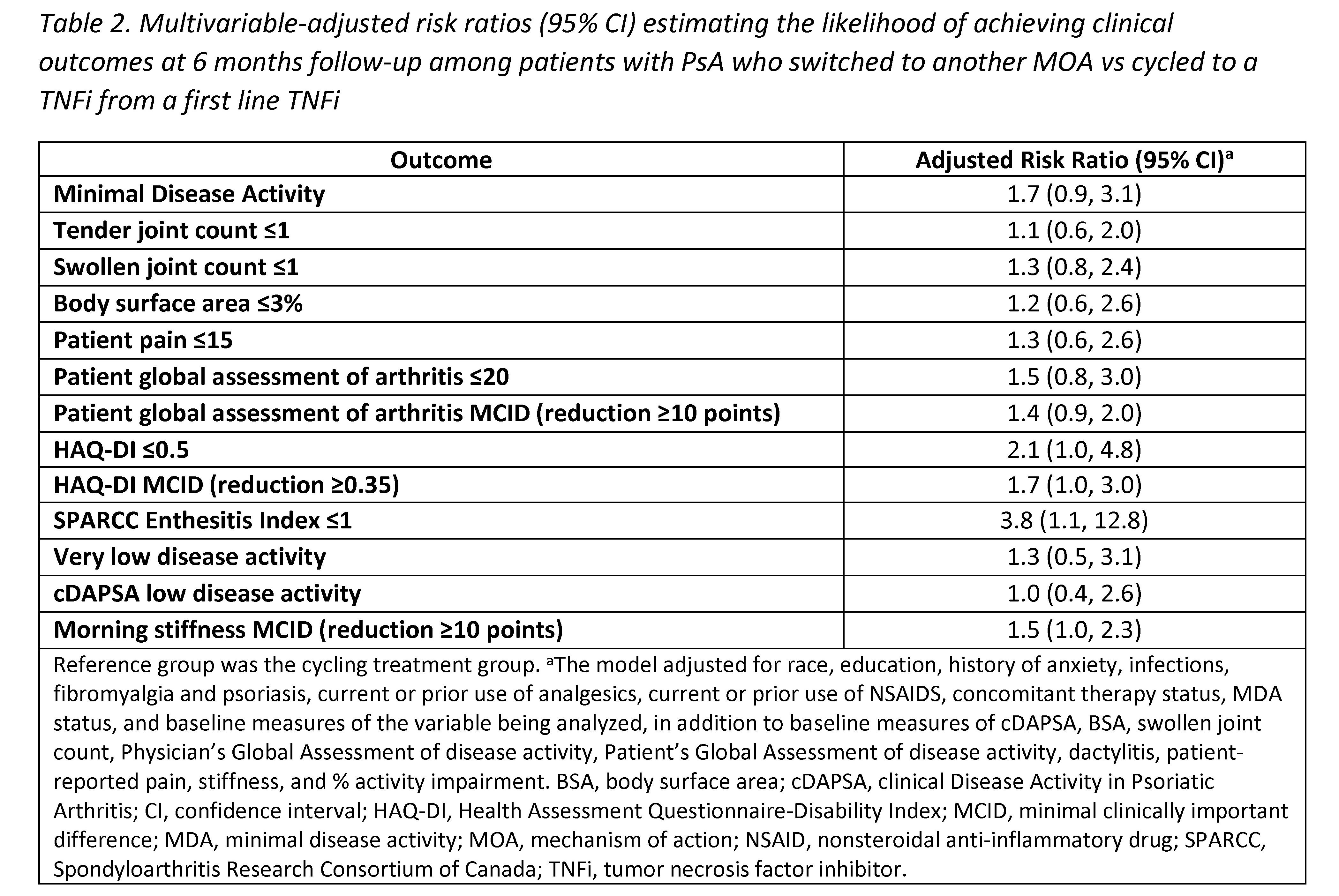
Results: Among 394 eligible pts initiating a 2nd line therapy, there were 205 (52%) cyclers and 189 (48%) switchers. Groups were similar at baseline in mean age (55 years) and gender (54% female) (Table 1). Cyclers were on 1st line TNFi therapy for a shorter duration vs switchers (11.4 vs 14.7 months, d=0.26). At baseline, switchers had greater severity of psoriasis (mean body surface area; 6.5 vs 4.7) and worse disease activity, with lower proportions in a state of minimal disease activity (MDA; 22% vs 28%) or low disease activity (clinical disease activity in PsA [cDAPSA] LDA; 26% vs 30%) compared to cyclers, though differences were small (all d< 0.2). At the 6-month follow-up, switchers trended towards having an increased likelihood of achieving all clinical outcomes vs cyclers, though most CIs included 1.0 (Table 2). Data suggest switchers had 70% greater likelihood of achieving MDA (RR [95% CI] = 1.7 [0.9, 3.1]) and a nearly 4 times higher likelihood of achieving a Spondyloarthritis Research Consortium of Canada Enthesitis index score ≤1 (3.8 [1.1, 12.8]). Switchers vs cyclers had 2 times the likelihood of achieving HAQ-DI ≤0.5 (2.1 [1.0, 4.8]) and a 30% greater likelihood of achieving a pt pain score ≤15 (1.3 [0.6, 2.6]); similarly, switchers had a greater likelihood of achieving MCID in HAQ-DI (1.7 [1.0, 3.0]), pt global assessment of arthritis (1.4 [0.9, 2.0]), and morning stiffness (1.5 [1.0, 2.3]) vs cyclers.
Conclusion: Our findings in this sample of PsA pts in a real-world setting suggest that switching to a different MOA may lead to comparable or better outcomes vs cycling among TNFi. While further confirmatory studies are warranted, our results indicate physicians may consider non-TNFi biologics as an appropriate option when pts discontinue 1st line TNFi therapy.
Disclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; R. McLean, CorEvitas; T. Blachley, CorEvitas; N. Middaugh, CorEvitas; M. Mittal, AbbVie; J. Clewell, AbbVie; S. Ciecinski, AbbVie; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.
Background/Purpose: While evidence in patients (pts) with RA suggests that switching to a therapy with a different mechanism of action (MOA) may be more effective than cycling among TNF inhibitors (TNFi) after discontinuing a 1st line TNFi, the relative effectiveness of cycling vs switching in pts with PsA is currently unknown. This study compared clinical outcomes between pts with PsA who initiated a TNFi vs a non-TNFi biologic after discontinuing a 1st line TNFi in a real-world setting.
Methods: Pts with a clinical diagnosis of PsA enrolled in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry who initiated a 2nd line advanced therapy (baseline) after discontinuation of 1st line TNFi between May 2013–January 2022 were included. Eligible pts had a follow-up visit 6-months after initiating the 2nd line therapy. Pts were stratified into two cohorts: 1) those who initiated a 2nd line TNFi (cyclers) and 2) those who initiated a 2nd line non-TNFi biologic (switchers). Baseline characteristics were summarized descriptively and compared between the groups using standardized differences (d; 0.2, 0.5, 0.8 considered small, medium, and large effects). Multivariable adjusted Poisson regression models were used to calculate risk ratios (RR) and 95% confidence intervals (CI) estimating the association between cycling vs switching status and clinical outcomes at 6 months.
Results: Among 394 eligible pts initiating a 2nd line therapy, there were 205 (52%) cyclers and 189 (48%) switchers. Groups were similar at baseline in mean age (55 years) and gender (54% female) (Table 1). Cyclers were on 1st line TNFi therapy for a shorter duration vs switchers (11.4 vs 14.7 months, d=0.26). At baseline, switchers had greater severity of psoriasis (mean body surface area; 6.5 vs 4.7) and worse disease activity, with lower proportions in a state of minimal disease activity (MDA; 22% vs 28%) or low disease activity (clinical disease activity in PsA [cDAPSA] LDA; 26% vs 30%) compared to cyclers, though differences were small (all d< 0.2). At the 6-month follow-up, switchers trended towards having an increased likelihood of achieving all clinical outcomes vs cyclers, though most CIs included 1.0 (Table 2). Data suggest switchers had 70% greater likelihood of achieving MDA (RR [95% CI] = 1.7 [0.9, 3.1]) and a nearly 4 times higher likelihood of achieving a Spondyloarthritis Research Consortium of Canada Enthesitis index score ≤1 (3.8 [1.1, 12.8]). Switchers vs cyclers had 2 times the likelihood of achieving HAQ-DI ≤0.5 (2.1 [1.0, 4.8]) and a 30% greater likelihood of achieving a pt pain score ≤15 (1.3 [0.6, 2.6]); similarly, switchers had a greater likelihood of achieving MCID in HAQ-DI (1.7 [1.0, 3.0]), pt global assessment of arthritis (1.4 [0.9, 2.0]), and morning stiffness (1.5 [1.0, 2.3]) vs cyclers.
Conclusion: Our findings in this sample of PsA pts in a real-world setting suggest that switching to a different MOA may lead to comparable or better outcomes vs cycling among TNFi. While further confirmatory studies are warranted, our results indicate physicians may consider non-TNFi biologics as an appropriate option when pts discontinue 1st line TNFi therapy.


Disclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; R. McLean, CorEvitas; T. Blachley, CorEvitas; N. Middaugh, CorEvitas; M. Mittal, AbbVie; J. Clewell, AbbVie; S. Ciecinski, AbbVie; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech.

