Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Treatment I: Switching or Discontinuation of Therapies (1585–1590
1588: Outcomes of Etanercept and Janus Kinase Inhibitor Treatment After First-line Use of Adalimumab in Patients with Rheumatoid Arthritis
Sunday, November 13, 2022
3:45 PM – 3:55 PM Eastern Time
Location: Room 120
- DP
Dimitrios Pappas, MD, MPH
CorEvitas
New York, NY, United States
Presenting Author(s)
Dimitrios Pappas1, Jacqueline O'Brien2, Lin Guo2, Ying Shan2, Joshua Baker3, Greg Kricorian4, Scott Stryker5 and David Collier6, 1Corrona Research Foundation; CorEvitas, LLC, Albany, NY, 2CorEvitas, LLC, Waltham, MA, 3University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, 4Amgen, Inc., Thousand Oaks, CA, 5Amgen Inc., San Francisco, CA, 6Amgen Inc., Simi Valley, CA
Background/Purpose: It is unclear whether a patient with rheumatoid arthritis (RA) whose disease fails to respond to 1 drug in a therapeutic class should switch to a different drug in the same class or switch to a different class. The objective of this analysis was to describe effectiveness and treatment patterns for patients who had prior treatment with adalimumab (ADA), a tumor necrosis factor inhibitor (TNFi), and switched to either etanercept (ETN) or a Janus kinase inhibitor (JAKi).
Methods: Data on second-line initiators of ETN or JAKis (tofacitinib, baricitinib, and upadacitinib) during or after November 2012 following first-line use of ADA were obtained from the prospective, multicenter, observational, disease-based CorEvitas RA Registry. First-line ADA use could have occurred before registry enrollment, and switching could occur at any time after initiation. We report reasons for ADA discontinuation and descriptive statistics at baseline (initiation of ETN or JAKis). Persistency of therapy was calculated. Effectiveness outcomes are presented as unadjusted mean changes and results from linear and logistic mixed-effects regression models adjusted for baseline covariates imbalanced at baseline. Outcomes are evaluated at 6 and 12 months.
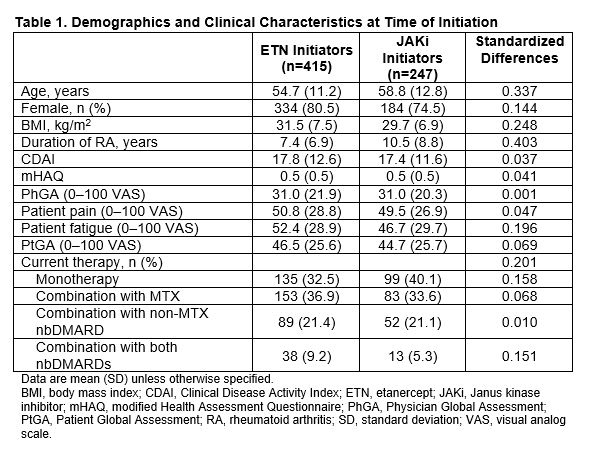
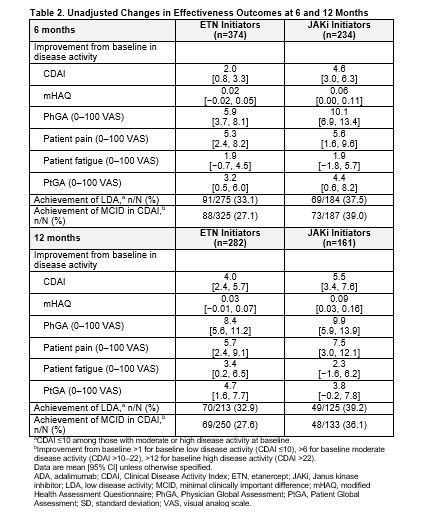
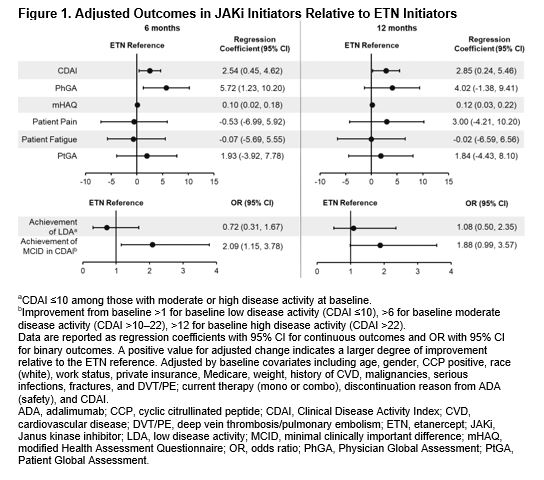
Results: Of all patients who had received first-line ADA and had a follow-up visit, 415 switched to ETN and 247 switched to a JAKi. The main reason for ADA discontinuation was lack of efficacy (50% for both groups) followed by safety (16.1% and 17.4% for ETN and JAKi initiators, respectively). The average duration of ADA treatment was ~1 year for both groups. Demographics and clinical characteristics were generally similar between groups with the exception that patients who switched to JAKis had longer duration of RA at baseline (Table 1). At 6 months, 60.2% and 76.9% remained on ETN and JAKi, respectively. At 12 months, 46.5% and 67.1% remained on ETN and JAKi, respectively. In unadjusted analyses, improvements in disease activity were seen in both groups (Table 2). In analyses adjusting for factors that were imbalanced at baseline, patients who switched from first-line ADA to a JAKi had greater improvement in Clinical Disease Activity Index (CDAI), Physician Global Assessment, and modified Health Assessment Questionnaire (mHAQ) scores and were more likely to achieve a minimal clinically important difference (MCID) in CDAI than patients who switched to ETN (Figure 1). However, there were no significant differences in change in pain, fatigue, Patient Global Assessment, or achievement of low disease activity between those who switched to ETN and those who switched to a JAKi (Figure 1). Patterns of results were similar at 6 and 12 months between JAKi initiators and ETN initiators.
Conclusion: In this population of patients with RA whose disease failed treatment with a first-line TNFi, improvements in disease activity and patient-reported outcomes were seen in patients who switched to ETN and in those who switched to a JAKi. Some measures of effectiveness showed greater improvement in the JAKi group. However, this real-world study demonstrates that some patients can benefit from TNF cycling. Further analyses will be needed to determine which subsets of patients benefit from TNFi vs JAKi therapy.
Disclosures: D. Pappas, CorEvitas, LLC, Corrona Research Foundation, AbbVie, Novartis, Roche Hellas, Sanofi; J. O'Brien, CorEvitas, LLC.; L. Guo, CorEvitas, LLC.; Y. Shan, CorEvitas, LLC.; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; G. Kricorian, Amgen; S. Stryker, Amgen; D. Collier, Amgen.
Background/Purpose: It is unclear whether a patient with rheumatoid arthritis (RA) whose disease fails to respond to 1 drug in a therapeutic class should switch to a different drug in the same class or switch to a different class. The objective of this analysis was to describe effectiveness and treatment patterns for patients who had prior treatment with adalimumab (ADA), a tumor necrosis factor inhibitor (TNFi), and switched to either etanercept (ETN) or a Janus kinase inhibitor (JAKi).
Methods: Data on second-line initiators of ETN or JAKis (tofacitinib, baricitinib, and upadacitinib) during or after November 2012 following first-line use of ADA were obtained from the prospective, multicenter, observational, disease-based CorEvitas RA Registry. First-line ADA use could have occurred before registry enrollment, and switching could occur at any time after initiation. We report reasons for ADA discontinuation and descriptive statistics at baseline (initiation of ETN or JAKis). Persistency of therapy was calculated. Effectiveness outcomes are presented as unadjusted mean changes and results from linear and logistic mixed-effects regression models adjusted for baseline covariates imbalanced at baseline. Outcomes are evaluated at 6 and 12 months.
Results: Of all patients who had received first-line ADA and had a follow-up visit, 415 switched to ETN and 247 switched to a JAKi. The main reason for ADA discontinuation was lack of efficacy (50% for both groups) followed by safety (16.1% and 17.4% for ETN and JAKi initiators, respectively). The average duration of ADA treatment was ~1 year for both groups. Demographics and clinical characteristics were generally similar between groups with the exception that patients who switched to JAKis had longer duration of RA at baseline (Table 1). At 6 months, 60.2% and 76.9% remained on ETN and JAKi, respectively. At 12 months, 46.5% and 67.1% remained on ETN and JAKi, respectively. In unadjusted analyses, improvements in disease activity were seen in both groups (Table 2). In analyses adjusting for factors that were imbalanced at baseline, patients who switched from first-line ADA to a JAKi had greater improvement in Clinical Disease Activity Index (CDAI), Physician Global Assessment, and modified Health Assessment Questionnaire (mHAQ) scores and were more likely to achieve a minimal clinically important difference (MCID) in CDAI than patients who switched to ETN (Figure 1). However, there were no significant differences in change in pain, fatigue, Patient Global Assessment, or achievement of low disease activity between those who switched to ETN and those who switched to a JAKi (Figure 1). Patterns of results were similar at 6 and 12 months between JAKi initiators and ETN initiators.
Conclusion: In this population of patients with RA whose disease failed treatment with a first-line TNFi, improvements in disease activity and patient-reported outcomes were seen in patients who switched to ETN and in those who switched to a JAKi. Some measures of effectiveness showed greater improvement in the JAKi group. However, this real-world study demonstrates that some patients can benefit from TNF cycling. Further analyses will be needed to determine which subsets of patients benefit from TNFi vs JAKi therapy.



Disclosures: D. Pappas, CorEvitas, LLC, Corrona Research Foundation, AbbVie, Novartis, Roche Hellas, Sanofi; J. O'Brien, CorEvitas, LLC.; L. Guo, CorEvitas, LLC.; Y. Shan, CorEvitas, LLC.; J. Baker, Bristol Myers Squibb, Pfizer, CorEvitas LLC, Burns-White, LLC, RediTrex; G. Kricorian, Amgen; S. Stryker, Amgen; D. Collier, Amgen.

