Back
Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes I: Diagnosis and Disease Activity (1609–1614)
1610: Are Serological Protease-mediated Peptides of Tissue Remodeling and Inflammation the New Disease Activity Biomarkers in Psoriatic Arthritis Patients?
Sunday, November 13, 2022
4:45 PM – 4:55 PM Eastern Time
Location: Room 113
- VC
Vinod Chandran, MD, PhD, MBBS, DM, FRCPC
University of Toronto
Toronto, ON, Canada
Presenting Author(s)
Solveig S. Groen1, Signe Holm Nielsen2, Anne-Christine Bay-Jensen2, Mozhgan Rasti3, Darshini Ganatra3, Katerina Oikonomopoulou3 and Vinod Chandran4, 1Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark, 2Nordic Bioscience, Herlev, Denmark, 3Schroeder Arthritis Institute, Krembil Research Institute, Toronto, ON, Canada, 4Departments of Medicine and Laboratory Medicine and Pathobiology, University of Toronto/ Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, Toronto, ON, Canada
Background/Purpose: Inflammatory and degenerative processes in the joint tissues are hallmarks of patients with psoriatic arthritis (PsA). Here, proteases play a major role in remodeling of the extracellular matrix (ECM) where collagens are major structural components. During inflammation, proteolytic processing of the ECM leads to unique protease-mediated breakdown products of collagens and proinflammatory effector molecules. These pathology-specific protein epitopes are released into the circulation and can be quantified in serum as biomarkers of tissue remodeling and inflammation. In the search for valid and clinically useful biomarkers, these protease-mediated peptides may be helpful in assessing disease activity objectively in PsA patients which can guide medical therapy and monitor treatment response. Our aim is to identify serum biomarkers associated with flares in PsA patients.
Methods: Sera were obtained at the Toronto Western Hospital, Canada, from PsA patients unselected for disease activity (n=99, mean ±SD age 45.5 ±12.4, 47% female, defined "PsA") and PsA patients with flares requiring knee joint synovial fluid aspiration and corticosteroid injection (n=32, mean ±SD age 45.6 ±12.9, 25% female, defined "PsA Flares"). ECM remodeling was quantified using serological fibrogenesis biomarkers of type III and VI collagen formation (PRO-C3, and PRO-C6 respectively), and catabolic biomarkers measuring type I, III, and VI collagen degradation (C1M, C3M, C6M respectively). Moreover, biomarkers of macrophage activity (citrullinated and MMP degraded vimentin; VICM) and neutrophil activity (human neutrophil elastase cleaved fragment of S100A9; CPa9-HNE), and tissue inflammation (MMP-mediated metabolite of C-reactive protein; CRPM) were measured. Statistically significant difference between the two groups was calculated by Mann-Whitney U test and a p-value below 0.05 was considered significant. ROC curve analysis was performed to describe the discrimination accuracy of each biomarker between the two patient groups.
Results: PsA patients with flares presented higher levels of type I and III collagen degradation with 37% and 20% (Fig. 1 A, B) and type III and VI collagen formation with 44% and 22% (Fig. 1 D, E) compared to patients in the PsA group with no flares. Biomarker levels reflecting macrophage and neutrophil activity including tissue damage were elevated with 50%, 60%, 37% in PsA with flares compared to PsA with no flares (Fig. 1 F, G, H). Among these biomarkers, the fibrotic marker PRO-C3 and the neutrophil activity marker CPa9-HNE, achieved the highest AUC of 0.86 (p< 0.0001) and 0.89 (p< 0.0001), respectively (Fig. 2 A, B).
Conclusion: Serum biomarkers of tissue remodeling and inflammation were elevated in PsA patients with flares compared to PsA patients with no flares. Biomarkers of neutrophil activity and fibrosis showed best discriminatory performance for separating PsA patients with flares from those with no flares and may serve as potential biomarkers of increased disease activity, as a result of inflammation-driven proteolysis in the joints.
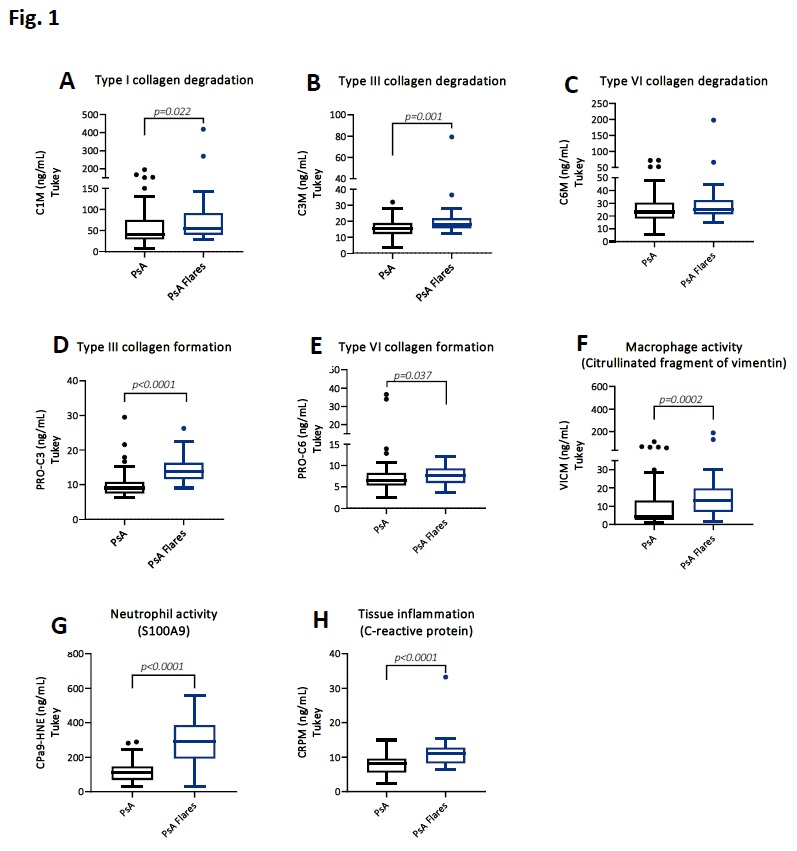 Serological biomarkers of tissue remodeling and inflammation in PsA patients with flares compared to PsA patients with no flares
Serological biomarkers of tissue remodeling and inflammation in PsA patients with flares compared to PsA patients with no flares
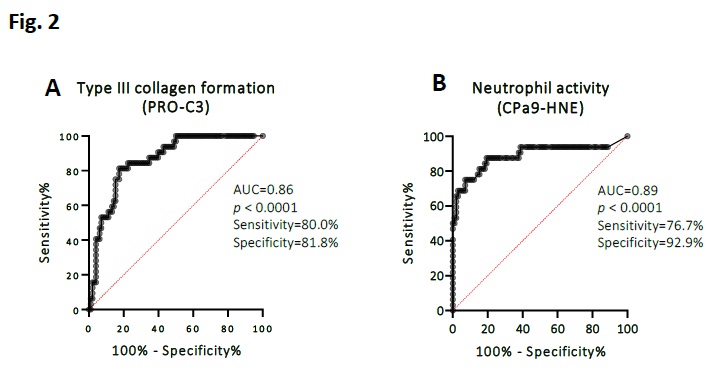 ROC curve analysis of the fibrotic marker PRO-C3 and the neutrophil activity marker CPa9-HNE
ROC curve analysis of the fibrotic marker PRO-C3 and the neutrophil activity marker CPa9-HNE
Disclosures: S. Groen, None; S. Holm Nielsen, Nordic Bioscience; A. Bay-Jensen, Nordic Bioscience; M. Rasti, None; D. Ganatra, None; K. Oikonomopoulou, None; V. Chandran, AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Amgen, Pfizer Inc, UCB.
Background/Purpose: Inflammatory and degenerative processes in the joint tissues are hallmarks of patients with psoriatic arthritis (PsA). Here, proteases play a major role in remodeling of the extracellular matrix (ECM) where collagens are major structural components. During inflammation, proteolytic processing of the ECM leads to unique protease-mediated breakdown products of collagens and proinflammatory effector molecules. These pathology-specific protein epitopes are released into the circulation and can be quantified in serum as biomarkers of tissue remodeling and inflammation. In the search for valid and clinically useful biomarkers, these protease-mediated peptides may be helpful in assessing disease activity objectively in PsA patients which can guide medical therapy and monitor treatment response. Our aim is to identify serum biomarkers associated with flares in PsA patients.
Methods: Sera were obtained at the Toronto Western Hospital, Canada, from PsA patients unselected for disease activity (n=99, mean ±SD age 45.5 ±12.4, 47% female, defined "PsA") and PsA patients with flares requiring knee joint synovial fluid aspiration and corticosteroid injection (n=32, mean ±SD age 45.6 ±12.9, 25% female, defined "PsA Flares"). ECM remodeling was quantified using serological fibrogenesis biomarkers of type III and VI collagen formation (PRO-C3, and PRO-C6 respectively), and catabolic biomarkers measuring type I, III, and VI collagen degradation (C1M, C3M, C6M respectively). Moreover, biomarkers of macrophage activity (citrullinated and MMP degraded vimentin; VICM) and neutrophil activity (human neutrophil elastase cleaved fragment of S100A9; CPa9-HNE), and tissue inflammation (MMP-mediated metabolite of C-reactive protein; CRPM) were measured. Statistically significant difference between the two groups was calculated by Mann-Whitney U test and a p-value below 0.05 was considered significant. ROC curve analysis was performed to describe the discrimination accuracy of each biomarker between the two patient groups.
Results: PsA patients with flares presented higher levels of type I and III collagen degradation with 37% and 20% (Fig. 1 A, B) and type III and VI collagen formation with 44% and 22% (Fig. 1 D, E) compared to patients in the PsA group with no flares. Biomarker levels reflecting macrophage and neutrophil activity including tissue damage were elevated with 50%, 60%, 37% in PsA with flares compared to PsA with no flares (Fig. 1 F, G, H). Among these biomarkers, the fibrotic marker PRO-C3 and the neutrophil activity marker CPa9-HNE, achieved the highest AUC of 0.86 (p< 0.0001) and 0.89 (p< 0.0001), respectively (Fig. 2 A, B).
Conclusion: Serum biomarkers of tissue remodeling and inflammation were elevated in PsA patients with flares compared to PsA patients with no flares. Biomarkers of neutrophil activity and fibrosis showed best discriminatory performance for separating PsA patients with flares from those with no flares and may serve as potential biomarkers of increased disease activity, as a result of inflammation-driven proteolysis in the joints.
 Serological biomarkers of tissue remodeling and inflammation in PsA patients with flares compared to PsA patients with no flares
Serological biomarkers of tissue remodeling and inflammation in PsA patients with flares compared to PsA patients with no flares ROC curve analysis of the fibrotic marker PRO-C3 and the neutrophil activity marker CPa9-HNE
ROC curve analysis of the fibrotic marker PRO-C3 and the neutrophil activity marker CPa9-HNEDisclosures: S. Groen, None; S. Holm Nielsen, Nordic Bioscience; A. Bay-Jensen, Nordic Bioscience; M. Rasti, None; D. Ganatra, None; K. Oikonomopoulou, None; V. Chandran, AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Amgen, Pfizer Inc, UCB.

