Back
Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – Non-ANCA-Associated and Related Disorders I (1615–1620)
1616: Drug Use and Pregnancy Outcomes in Pregnant Women with Systemic Vasculitis: A Nation Wide Cohort Study
Sunday, November 13, 2022
4:45 PM – 4:55 PM Eastern Time
Location: Ballroom AB
- CM
Camille Mettler, MD
APHP, Service de Médecine Interne, Hôpital Cochin
Paris, France
Presenting Author(s)
Camille Mettler1, Nathanaël Beeker2, Mathis Collier2, Veronique Le Guern3, Benjamin Terrier4 and Laurent Chouchana5, 1Department of Internal Medicine, National Referral Center for Rare Systemic and Autoimmune Diseases, Cochin Hospital, Paris, France, 2Unité de Recherche clinique, Hôpital Cochin, Assistance Publique-Hôpitaux de Paris, Paris, France, 3Hôpital Cochin, Paris, France, 4National Referral Center for Rare Systemic Autoimmune Diseases, Cochin Hospital, Paris, France, 5Pharmacology department, Centre Régional de Pharmacovigilance, Hôpital Cochin, Assistance Publique-Hôpitaux de Paris, Paris, France
Background/Purpose: Women of childbearing age are rarely affected by systemic vasculitis (SV), explaining the lack of solid data regarding pregnancy in these patients. The objective of this work was to describe pregnancy course in women with (SV), exposure to immunosuppressive drugs, and obstetric events such as preterm birth (PTB) and hypertensive complications of pregnancy.
Methods: We performed two analyses using the national cohort of pregnant women with SV from the French National Health Data System. Immunosuppressant exposure was identified from outpatient and inpatient reimbursement data. Pregnancies among women with SV (cases) were matched with pregnancies without SV (controls) using a 1:5 case-control ratio. We estimated the risk of PTB and hypertensive disorders during pregnancy among women with SV by comparison with matched population and we investigated associated risk factors. SV flare during a trimester was defined as an augmentation of glucocorticoids (GCs) daily dose.
Results: Of 3,246,454 pregnancies, 649 pregnancies were observed in 606 women with SV. Immunosuppressant and GCs use decreased before pregnancy and then increased after pregnancy (48.4%, 40.7%, 50.4%, respectively before, during, after) (Figure 1). Prevalence of GCs use was broadly stable during pregnancy from 27.9% to 27.6% and 23.7% in the 1st, 2nd and 3rd trimesters, respectively, with a daily dose of 5 mg. The number of patients treated with non-recommended immunosuppressant during pregnancy gradually decreased before pregnancy and then increased after delivery, whereas proportion of SV flare did not increase significantly during pregnancy (Figure 2). SV were significantly associated with PTB (OR 1.8, 95% CI [1.4-2.3]) and hypertensive disorders (OR 1.7, 95% CI [1.3-2.2]) (Table 1). Subgroup of SV significantly associated with PTB and hypertensive disorders were ANCA associated vasculitis (AAV), large vessel vasculitis (LVV), and immune complex small vessel vasculitis (ICV). Maternal age, end-stage renal disease before pregnancy and the occurrence of a vasculitis flare during pregnancy were independently associated with the occurrence of PTB. End-stage renal disease before pregnancy, hypertension before pregnancy, the occurrence of a vasculitis flare during pregnancy and the subgroup of SV were independently associated with the occurrence of hypertensive disorders.
Conclusion: Our data suggest vasculitis control during pregnancy with anticipation and cessation of teratogenic drugs before the beginning of the pregnancy. PTB and hypertensive disorders of pregnancy were more frequent among women presenting SV compared to a matched population and occurrence of vasculitis flare was associated with these complications. Our results support the importance of preconception consultations and then specialized follow-up of these patients in order to review treatments, replace non-recommended and/or teratogenic drugs with drugs considered safe during pregnancy, as well as ensure stability of the underlying disease.
.jpg) Figure 1. Pattern of immunosuppressive drug use before, during and after pregnancy, in women with systemic vasculitis.
Figure 1. Pattern of immunosuppressive drug use before, during and after pregnancy, in women with systemic vasculitis.
Footnote: Panel A present pattern of immunosuppressive drugs use in systemic vasculitis overall; panel B present pattern of immunosuppressive drugs use according to systemic vasculitis subgroup.
Abbreviations: ACN or mTORi, Anticalcineurin and mTOR inhibitors; AAV, ANCA associated vasculitis; AZA, Azathioprine; GC, Glucocorticoids; ICV, Immune Complex Small Vessel Vasculitis; IM, Immunomodulatory; LVV, Large Vessel Vasculitis ; MMF, Mycophenolate mofetil; MTX, Methotrexate; MVV, Medium Vessel Vasculitis ; Other IS or Bio, Other immunosuppressive drugs or biologics; PostT1, PostT2, PostT3 and PostT4, 1st trimester, 2nd trimester, 3rd trimester and, 4th trimester after delivery, respectively; PreT1, PreT2, PreT3, PreT4, 1st trimester, 2nd trimester, 3rd trimester and, 4th trimester before pregnancy, respectively; T1, T2, T3, 1st trimester, 2nd trimester, and 3rd trimester of pregnancy, respectively; USV, Unclassified systemic vasculitis; VVV, Variable Vessel Vasculitis.
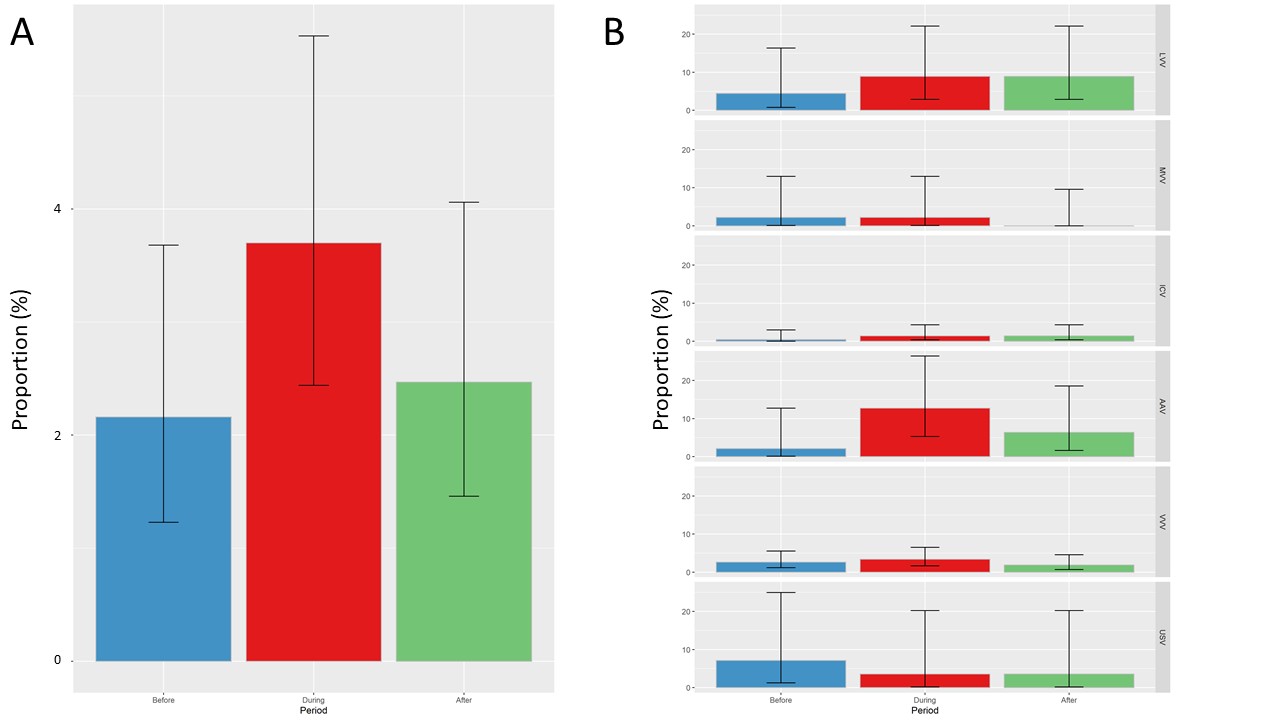 Figure 2. Proportion of systemic vasculitis flares during pregnancy
Figure 2. Proportion of systemic vasculitis flares during pregnancy
Footnote: Panel A presents proportion of systemic vasculitis flares in overall population; panel B presents proportion of disease flares according to systemic vasculitis subgroup.
Abbreviations: AAV, ANCA associated vasculitis; ICV, Immune Complex Small Vessel Vasculitis; LVV, Large Vessel Vasculitis; MVV, Medium Vessel Vasculitis; USV, Unclassified systemic vasculitis; VVV, Variable Vessel Vasculitis.
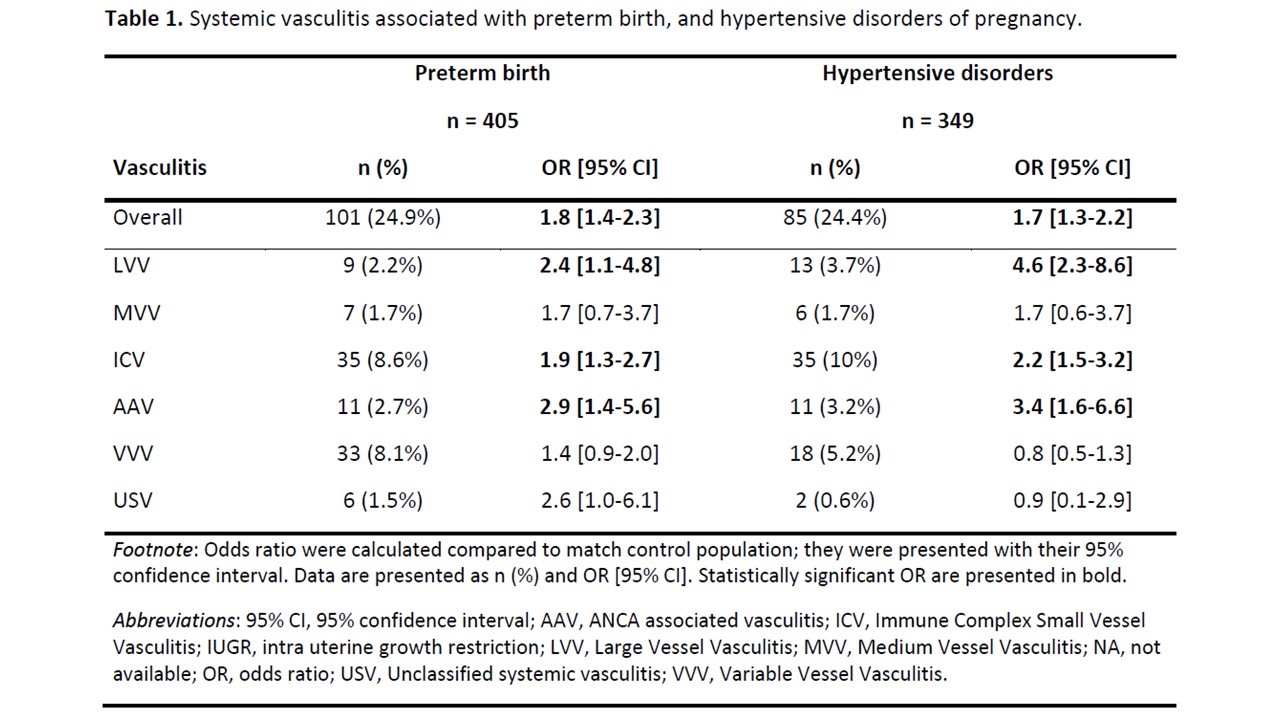 Table 1. Systemic vasculitis associated with preterm birth, and hypertensive disorders of pregnancy.
Table 1. Systemic vasculitis associated with preterm birth, and hypertensive disorders of pregnancy.
Disclosures: C. Mettler, None; N. Beeker, None; M. Collier, None; V. Le Guern, None; B. Terrier, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb(BMS), Eli Lilly, LFB, Boehinger Ingelheim, Vifor Pharma, Pfizer, Roche; L. Chouchana, None.
Background/Purpose: Women of childbearing age are rarely affected by systemic vasculitis (SV), explaining the lack of solid data regarding pregnancy in these patients. The objective of this work was to describe pregnancy course in women with (SV), exposure to immunosuppressive drugs, and obstetric events such as preterm birth (PTB) and hypertensive complications of pregnancy.
Methods: We performed two analyses using the national cohort of pregnant women with SV from the French National Health Data System. Immunosuppressant exposure was identified from outpatient and inpatient reimbursement data. Pregnancies among women with SV (cases) were matched with pregnancies without SV (controls) using a 1:5 case-control ratio. We estimated the risk of PTB and hypertensive disorders during pregnancy among women with SV by comparison with matched population and we investigated associated risk factors. SV flare during a trimester was defined as an augmentation of glucocorticoids (GCs) daily dose.
Results: Of 3,246,454 pregnancies, 649 pregnancies were observed in 606 women with SV. Immunosuppressant and GCs use decreased before pregnancy and then increased after pregnancy (48.4%, 40.7%, 50.4%, respectively before, during, after) (Figure 1). Prevalence of GCs use was broadly stable during pregnancy from 27.9% to 27.6% and 23.7% in the 1st, 2nd and 3rd trimesters, respectively, with a daily dose of 5 mg. The number of patients treated with non-recommended immunosuppressant during pregnancy gradually decreased before pregnancy and then increased after delivery, whereas proportion of SV flare did not increase significantly during pregnancy (Figure 2). SV were significantly associated with PTB (OR 1.8, 95% CI [1.4-2.3]) and hypertensive disorders (OR 1.7, 95% CI [1.3-2.2]) (Table 1). Subgroup of SV significantly associated with PTB and hypertensive disorders were ANCA associated vasculitis (AAV), large vessel vasculitis (LVV), and immune complex small vessel vasculitis (ICV). Maternal age, end-stage renal disease before pregnancy and the occurrence of a vasculitis flare during pregnancy were independently associated with the occurrence of PTB. End-stage renal disease before pregnancy, hypertension before pregnancy, the occurrence of a vasculitis flare during pregnancy and the subgroup of SV were independently associated with the occurrence of hypertensive disorders.
Conclusion: Our data suggest vasculitis control during pregnancy with anticipation and cessation of teratogenic drugs before the beginning of the pregnancy. PTB and hypertensive disorders of pregnancy were more frequent among women presenting SV compared to a matched population and occurrence of vasculitis flare was associated with these complications. Our results support the importance of preconception consultations and then specialized follow-up of these patients in order to review treatments, replace non-recommended and/or teratogenic drugs with drugs considered safe during pregnancy, as well as ensure stability of the underlying disease.
.jpg) Figure 1. Pattern of immunosuppressive drug use before, during and after pregnancy, in women with systemic vasculitis.
Figure 1. Pattern of immunosuppressive drug use before, during and after pregnancy, in women with systemic vasculitis. Footnote: Panel A present pattern of immunosuppressive drugs use in systemic vasculitis overall; panel B present pattern of immunosuppressive drugs use according to systemic vasculitis subgroup.
Abbreviations: ACN or mTORi, Anticalcineurin and mTOR inhibitors; AAV, ANCA associated vasculitis; AZA, Azathioprine; GC, Glucocorticoids; ICV, Immune Complex Small Vessel Vasculitis; IM, Immunomodulatory; LVV, Large Vessel Vasculitis ; MMF, Mycophenolate mofetil; MTX, Methotrexate; MVV, Medium Vessel Vasculitis ; Other IS or Bio, Other immunosuppressive drugs or biologics; PostT1, PostT2, PostT3 and PostT4, 1st trimester, 2nd trimester, 3rd trimester and, 4th trimester after delivery, respectively; PreT1, PreT2, PreT3, PreT4, 1st trimester, 2nd trimester, 3rd trimester and, 4th trimester before pregnancy, respectively; T1, T2, T3, 1st trimester, 2nd trimester, and 3rd trimester of pregnancy, respectively; USV, Unclassified systemic vasculitis; VVV, Variable Vessel Vasculitis.
 Figure 2. Proportion of systemic vasculitis flares during pregnancy
Figure 2. Proportion of systemic vasculitis flares during pregnancyFootnote: Panel A presents proportion of systemic vasculitis flares in overall population; panel B presents proportion of disease flares according to systemic vasculitis subgroup.
Abbreviations: AAV, ANCA associated vasculitis; ICV, Immune Complex Small Vessel Vasculitis; LVV, Large Vessel Vasculitis; MVV, Medium Vessel Vasculitis; USV, Unclassified systemic vasculitis; VVV, Variable Vessel Vasculitis.
 Table 1. Systemic vasculitis associated with preterm birth, and hypertensive disorders of pregnancy.
Table 1. Systemic vasculitis associated with preterm birth, and hypertensive disorders of pregnancy.Disclosures: C. Mettler, None; N. Beeker, None; M. Collier, None; V. Le Guern, None; B. Terrier, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb(BMS), Eli Lilly, LFB, Boehinger Ingelheim, Vifor Pharma, Pfizer, Roche; L. Chouchana, None.

