Back
Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Treatment II: Pre- and Early Disease (1603–1607)
1605: Profound Anticoagulant Effects of Initial Antirheumatic Treatments in Early Rheumatoid Arthritis Patients; A NORD-STAR Spin-off Study
Sunday, November 13, 2022
5:00 PM – 5:10 PM Eastern Time
Location: Room 121
- BD
Bas Dijkshoorn, MD, MSc
Reade
Amsterdam, Netherlands
Presenting Author(s)
Bas Dijkshoorn1, Daisy Vedder1, Anna Rudin2, Dan Nordstrom3, Bjorn Gudbjornsson4, Kristina Lend5, Till Uhlig6, Espen Haavardsholm6, Gerdur Maria Grondal7, Merete L Hetland8, Marte Heiberg6, Mikkel Østergaard9, Kim Horslev-Petersen10, Jon Lampa11, Ronald van Vollenhoven12, Aleksandra antovic13 and Michael Nurmohamed14, 1Reade Rheumatology Center, Amsterdam, Netherlands, 2Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy of University of Gothenburg, Gothenburg, Sweden, 3Helsinki University Hospital, Helsinki, Finland, 4Centre for Rheumatology Research, University Hospital, Reykjavik, Iceland, 5The Karolinska Institute, Stockholm, Sweden, 6Diakonhjemmet Hospital, Oslo, Norway, 7Landspitali University Hospital, Centre for Rheumatology Research, Reykjavik, Iceland, 8Rigshospitalet, Glostrup, Denmark, 9Rigshospitalet, University of Copenhagen, Glostrup, Denmark, 10Danish Hospital for Rheumatic Diseases, Haderslev, Denmark, 11Department of Medicine, Rheumatology Unit, Center for Molecular Medicine (CMM), Karolinska Institutet, Stockholm, Sweden; Department of Gastroenterology, Dermatology and Rheumatology, Karolinska University Hospital, Stockholm, Sweden, 12Amsterdam University Medical Centers, Amsterdam, Netherlands, 13Karolinska Institute, Department of Medicine Solna, Division of Rheumatology, Stockholm, Sweden, 14Amsterdam University Medical Center, Kortenhoef, Netherlands
Background/Purpose: Patients with rheumatoid arthritis (RA) are at an increased risk of venous thromboembolism. Thus far, there have not been any comparative studies investigating the effects of initial antirheumatic treatments in (very) early RA patients. Therefore our objective was to assess the effects of different initial treatments on hemostatic parameters in patients with early RA.
Methods: NORD-STAR is an international, multicentre, open-label, assessor-blinded, phase 4 study where patients with newly diagnosed RA started methotrexate (MTX) and were randomised 1:1:1:1 to a) conventional treatment (either prednisolone tapered to 5mg/day, or sulfasalazine combined with hydroxychloroquine and intra-articular corticosteroids), b) certolizumab pegol, c) abatacept, d) tocilizumab. This study is a spin-off from the main NORD-STAR study extensively investigating hemostatic system in 24 per protocol consecutive Dutch participants at baseline, 12 weeks and 24 weeks after the start of the treatment. Statistical analysis was done using paired samples t-test in SPSS version 28.
Results: The mean age of investigated patients was 51.8 (± 12.7) years and 58.3% were female. At baseline patients had an average DAS28 score of 4.6 (± 0.9) and had elevated levels of investigated coagulation biomarkers: Factor 1+2, fibrinogen, D-dimer and parameters of the two global hemostatic assays, i.e. endogenous thrombin potential (ETP) and overall hemostasis potential (OHP). These biomarkers decreased significantly at 12 and 24 weeks in patients in all groups (Table 1). Overall fibrinolytic potential (OFP) was decreased and clot lysis time (CLT) was prolonged at baseline, demonstrating impaired fibrinolytic activity in early RA. The reduction of coagulation parameters was significantly higher in biological treatment arms in comparison to the standard MTX treatment arm. In addition, tocilizumab was more effective compared to certolizumab and abatacept, (Figure 1), which was expected considering the direct inhibitory effect of this drug on the IL-6 synthesis and consequently the coagulation activation as well. After 24 weeks of treatment with methotrexate and tocilizumab, the average fibrinogen of patients was reduced by 63% vs 31% and 36% in the certolizumab and abatacept groups, respectively. The changes in DAS-28 and the changes in fibrinogen had a correlation of 0.385 which did not reach statistical significance.
Conclusion: Our results indicate an enhanced coagulation and fibrinolytic impairment in newly diagnosed RA patients. Effective antirheumatic treatments reduce this hemostatic imbalance, with significantly more pronounced effects of biologic drugs compared to conventional (MTX+glucocorticoids) treatment.
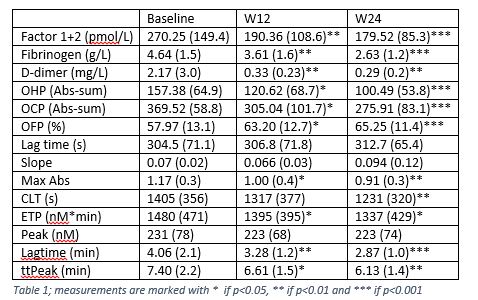
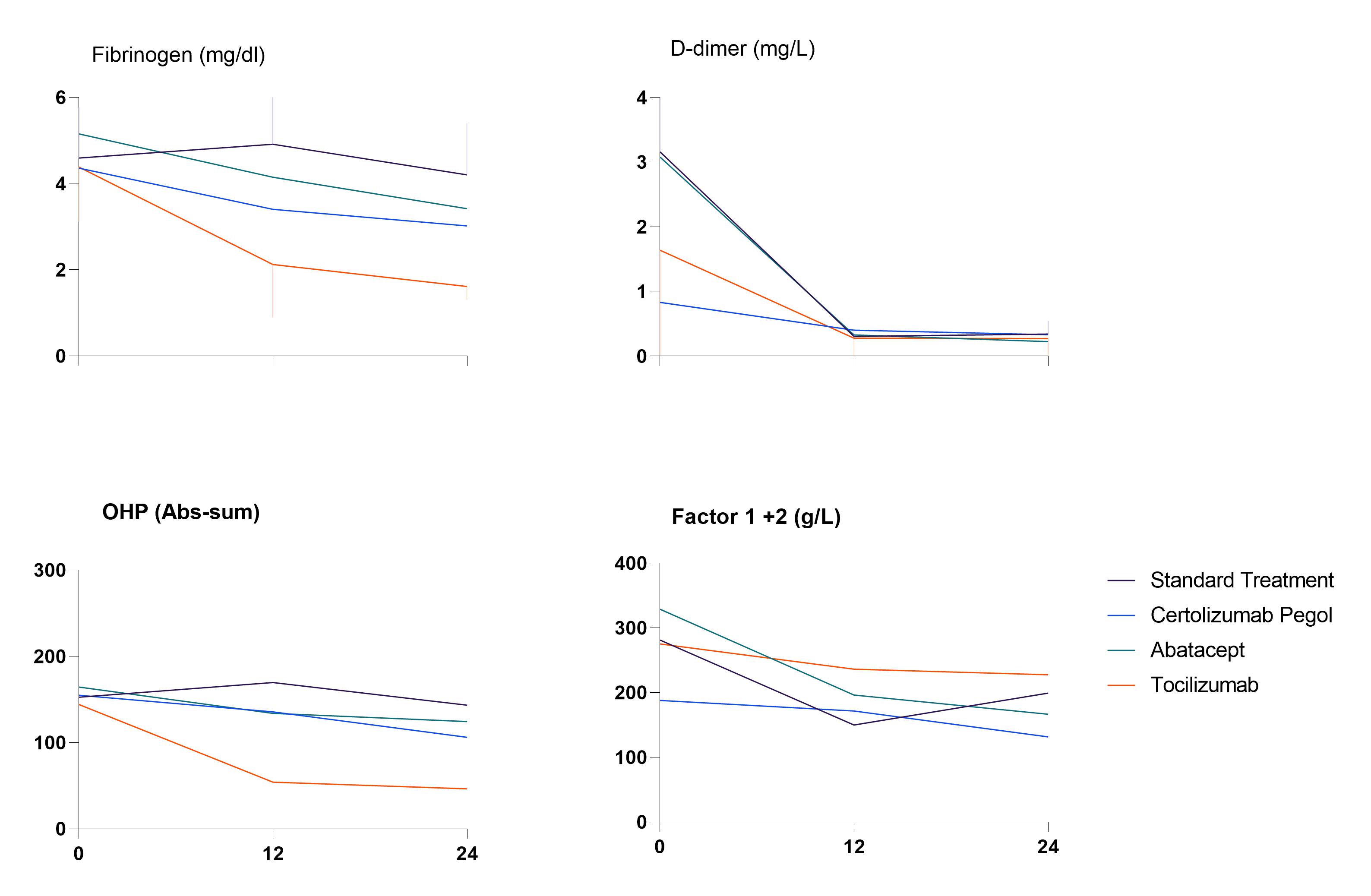 Figure (1), differences in coagulation factors between different treatment groups
Figure (1), differences in coagulation factors between different treatment groups
Disclosures: B. Dijkshoorn, None; D. Vedder, None; A. Rudin, None; D. Nordstrom, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Eli Lilly, Novartis, Pfizer, Roche, UCB; B. Gudbjornsson, Novartis, Amgen; K. Lend, None; T. Uhlig, None; E. Haavardsholm, Pfizer, AbbVie, Eli Lilly, UCB, Boehringer-Ingelheim, Gilead; G. Grondal, None; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac; M. Heiberg, None; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; K. Horslev-Petersen, None; J. Lampa, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; A. antovic, None; M. Nurmohamed, AbbVie/Abbott.
Background/Purpose: Patients with rheumatoid arthritis (RA) are at an increased risk of venous thromboembolism. Thus far, there have not been any comparative studies investigating the effects of initial antirheumatic treatments in (very) early RA patients. Therefore our objective was to assess the effects of different initial treatments on hemostatic parameters in patients with early RA.
Methods: NORD-STAR is an international, multicentre, open-label, assessor-blinded, phase 4 study where patients with newly diagnosed RA started methotrexate (MTX) and were randomised 1:1:1:1 to a) conventional treatment (either prednisolone tapered to 5mg/day, or sulfasalazine combined with hydroxychloroquine and intra-articular corticosteroids), b) certolizumab pegol, c) abatacept, d) tocilizumab. This study is a spin-off from the main NORD-STAR study extensively investigating hemostatic system in 24 per protocol consecutive Dutch participants at baseline, 12 weeks and 24 weeks after the start of the treatment. Statistical analysis was done using paired samples t-test in SPSS version 28.
Results: The mean age of investigated patients was 51.8 (± 12.7) years and 58.3% were female. At baseline patients had an average DAS28 score of 4.6 (± 0.9) and had elevated levels of investigated coagulation biomarkers: Factor 1+2, fibrinogen, D-dimer and parameters of the two global hemostatic assays, i.e. endogenous thrombin potential (ETP) and overall hemostasis potential (OHP). These biomarkers decreased significantly at 12 and 24 weeks in patients in all groups (Table 1). Overall fibrinolytic potential (OFP) was decreased and clot lysis time (CLT) was prolonged at baseline, demonstrating impaired fibrinolytic activity in early RA. The reduction of coagulation parameters was significantly higher in biological treatment arms in comparison to the standard MTX treatment arm. In addition, tocilizumab was more effective compared to certolizumab and abatacept, (Figure 1), which was expected considering the direct inhibitory effect of this drug on the IL-6 synthesis and consequently the coagulation activation as well. After 24 weeks of treatment with methotrexate and tocilizumab, the average fibrinogen of patients was reduced by 63% vs 31% and 36% in the certolizumab and abatacept groups, respectively. The changes in DAS-28 and the changes in fibrinogen had a correlation of 0.385 which did not reach statistical significance.
Conclusion: Our results indicate an enhanced coagulation and fibrinolytic impairment in newly diagnosed RA patients. Effective antirheumatic treatments reduce this hemostatic imbalance, with significantly more pronounced effects of biologic drugs compared to conventional (MTX+glucocorticoids) treatment.

 Figure (1), differences in coagulation factors between different treatment groups
Figure (1), differences in coagulation factors between different treatment groupsDisclosures: B. Dijkshoorn, None; D. Vedder, None; A. Rudin, None; D. Nordstrom, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Merck/MSD, Eli Lilly, Novartis, Pfizer, Roche, UCB; B. Gudbjornsson, Novartis, Amgen; K. Lend, None; T. Uhlig, None; E. Haavardsholm, Pfizer, AbbVie, Eli Lilly, UCB, Boehringer-Ingelheim, Gilead; G. Grondal, None; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac; M. Heiberg, None; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; K. Horslev-Petersen, None; J. Lampa, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; A. antovic, None; M. Nurmohamed, AbbVie/Abbott.

