Back
Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – Non-ANCA-Associated and Related Disorders I (1615–1620)
1620: Probability-based Diagnostic Algorithm in Suspected Giant Cell Arteritis: A Prospective, Multicentre Validity Data from HAS GCA Study
Sunday, November 13, 2022
5:45 PM – 5:55 PM Eastern Time
Location: Ballroom AB
- AS
Alwin Sebastian, MD, MSc, MRCP
University Hospital Limerick
Patrickswell, Limerick, Ireland
Presenting Author(s)
Alwin Sebastian1, alessandro tomelleri2, PIERLUIGI MACCHIONI3, Giulia Klinowski3, Carlo Salvarani4, Abdul Kayani5, Mohammad Tariq5, Diana Prieto-Peña6, Edoardo Conticini7, Muhammad Khurshid8, Sue Inness9, Jo Jackson9, Kornelis van der Geest10 and Bhaskar Dasgupta5, 1University Hospital Limerick, Dooradoyle, Ireland, 2IRCCS San Raffaele Hospital. Vita-Salute San Raffaele University, Milano, Italy, 3IRCCS-S.Maria Nuova, Reggio Emilia, Italy, 4Azienda USL -IRCCS di Reggio Emilia and Università di Modena e Reggio Emilia, Reggio Emilia, Reggio Emilia, Italy, 5Mid and South Essex University Hospital Groups, Southend, United Kingdom, 6Research Group on Genetic Epidemiology and Atherosclerosis in Systemic Diseases and in Metabolic Bone Diseases of the Musculoskeletal System, IDIVAL; and Department of Rheumatology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 7Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy, 8University Hospital Dorset, NHS foundation trust, UK, Poole, United Kingdom, 9University of Essex, School of Sport, Rehabilitation and Exercise science, Colchester, United Kingdom, 10University Medical Center Groningen, Groningen, Netherlands
Background/Purpose: The presentation of new-onset giant cell arteritis (GCA) is highly variable. It is vital to make a secure diagnosis to minimise the risk for visual loss, while excluding the mimicking conditions to avoid unnecessary glucocorticoid exposure. Although imaging tests (e.g., ultrasonography) are increasingly used in case of suspected GCA, the assessment of the clinical probability based on symptoms, signs and lab findings remains crucial. To standardize the estimation of clinical probability, the Southend pre-test probability score (SPTPS) has been recently developed. The SPTPS stratifies patients into low-risk, intermediate-risk and high-risk categories1,2. Retrospective studies from many centres3-6 have externally validated this SPTPS. The current study aimed to validate the SPTPS in a multicentre, prospective study.
Methods: This prospective, multicentre, observational study includes consecutive patients with suspected new-onset GCA recruited at 7 European centres participating in the HAS GCA study. SPTPS was calculated, and patients were stratified into the three risk categories: low-risk < 9, intermediate-risk 9-12 and high-risk >12. All patients underwent vascular ultrasonography (bilateral temporal arteries; common, parietal, frontal branches and axillary arteries). Vascular ultrasonography was considered positive for GCA when the intimal medial thickness is >0.42mm in common temporal, >0.29mm in Parietal, >0.34mm in frontal and >1.0mm in axillary arteries. Additional tests such as temporal artery biopsy, FDG-PET/CT, or CTA were performed at the treating physician's discretion. The final diagnosis was confirmed after 6 months of follow up.
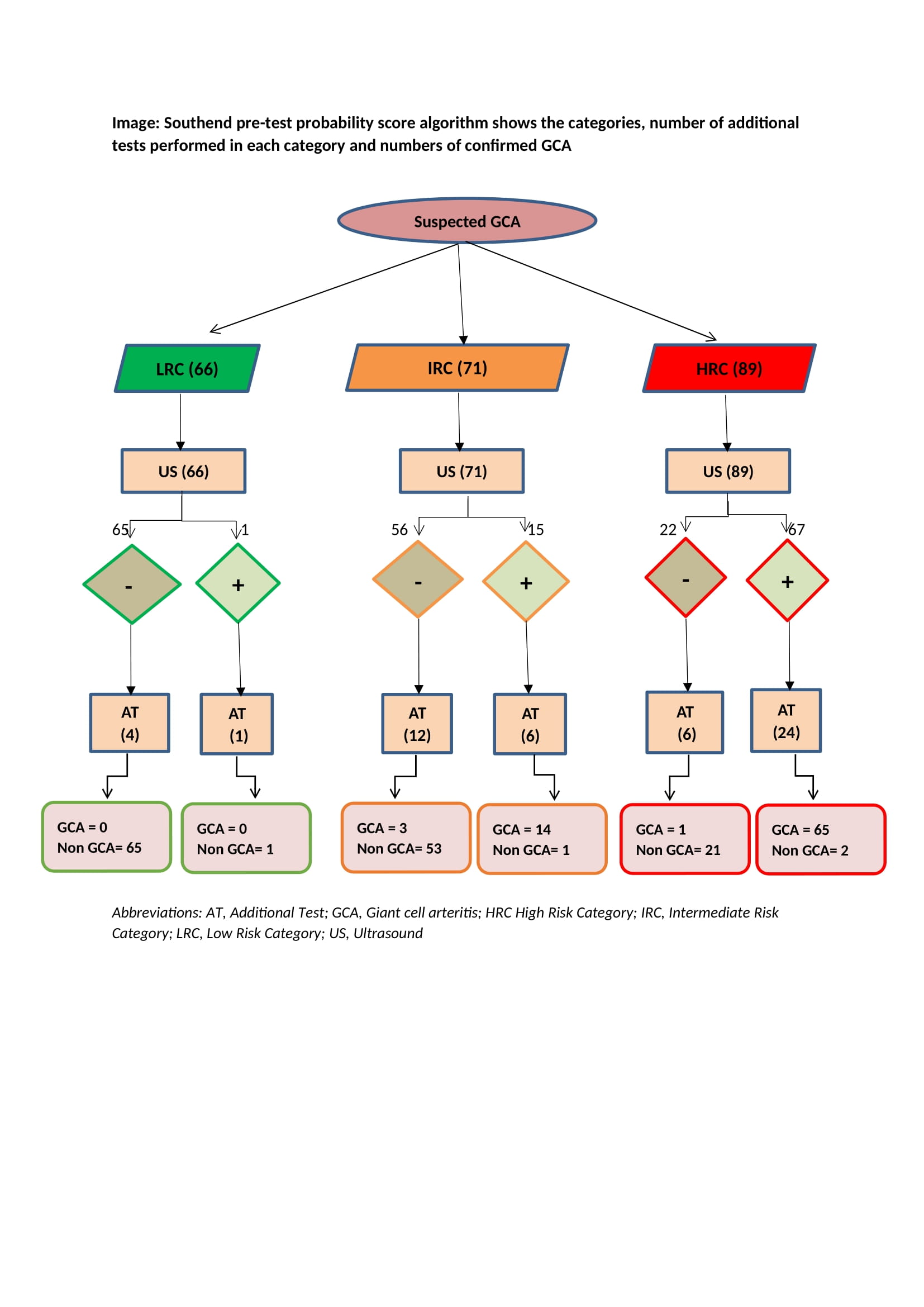
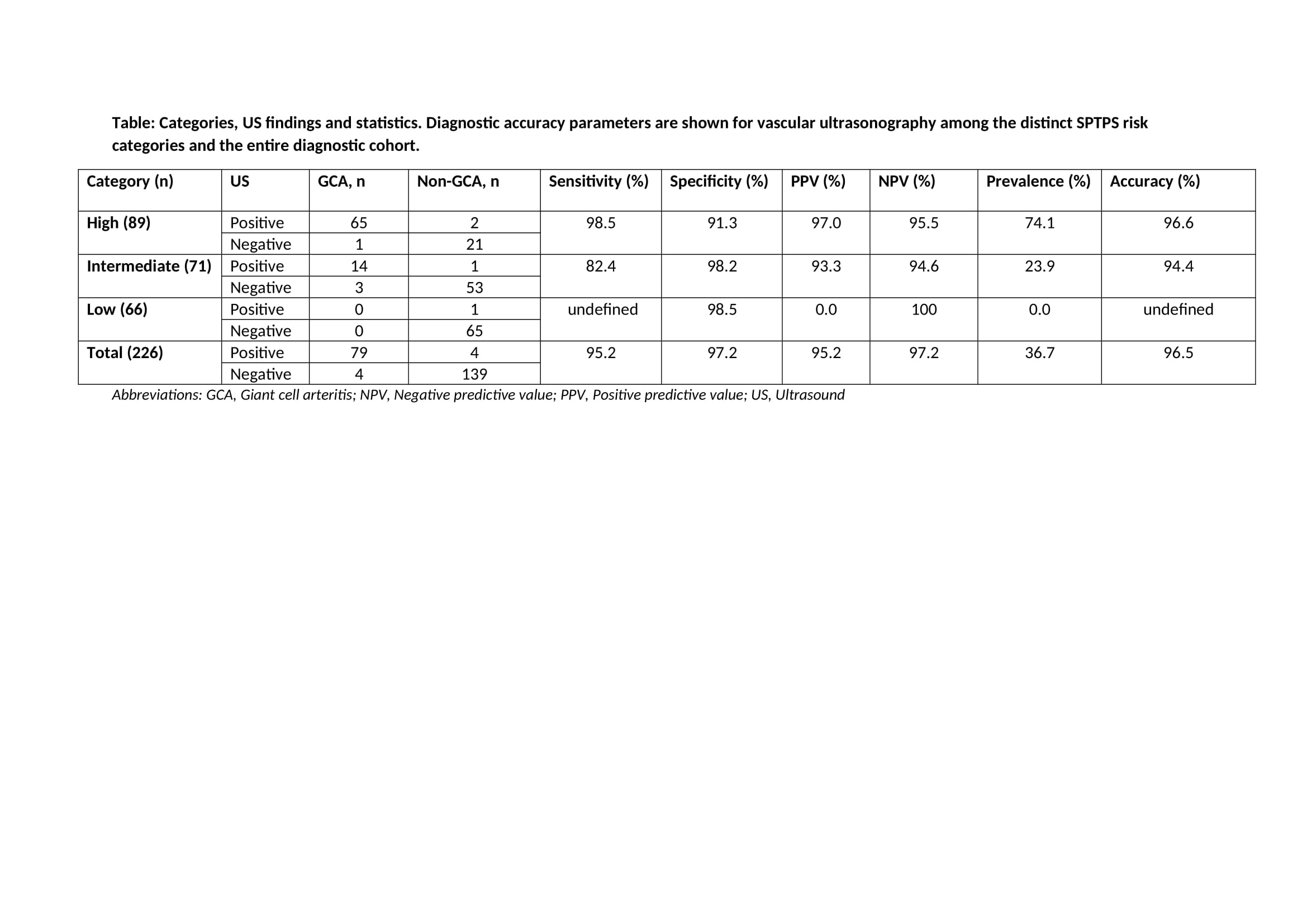
Results: A total of 226 patients were included in the study. A diagnosis of GCA was confirmed in 83 (36.73%) patients. SPTPS was low-risk in 66 (29.2%) patients, intermediate-risk in71 (31.4%) patients and high-risk in 89 (39.4%) patients (Image). The number of patients with GCA among patients in distinct risk categories was 0 (0%) in low-risk patients, 17 (20.5%) in intermediate-risk patients and 66 (79.5%) in high-risk patients. Among the three risk categories, high diagnostic accuracy was observed for ultrasonography (table).
Conclusion: The SPTPS is a useful tool for assessing the clinical probability of GCA among patients with suspected new-onset GCA. A combination of the SPTPS and vascular ultrasonography accurately discriminated between patients with and without GCA in our prospective, multicentre study.
References:
1. Laskou F, et al. Clin Exp Rheumatol. 2019 Feb 15
2. Sebastian A, et al. RMD Open. 2020;6(3)
3. Melville et al. Rheum Adv in Practice, Volume 6, Issue 1, 2022, rkab102
4. Oshinsky et al. Arthritis Rheumatol. 2021; 73 (suppl 10)
5. Mathake et al. Arthritis Rheumatol. 2021; 73 (suppl 10)
6. Fernández-Fernández et al. RMD Open 2022 Apr;8(1):e002120
Disclosures: A. Sebastian, None; a. tomelleri, None; P. MACCHIONI, None; G. Klinowski, None; C. Salvarani, None; A. Kayani, None; M. Tariq, None; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; E. Conticini, None; M. Khurshid, None; S. Inness, None; J. Jackson, None; K. van der Geest, Roche; B. Dasgupta, Roche, AbbVie/Abbott, sanofi.
Background/Purpose: The presentation of new-onset giant cell arteritis (GCA) is highly variable. It is vital to make a secure diagnosis to minimise the risk for visual loss, while excluding the mimicking conditions to avoid unnecessary glucocorticoid exposure. Although imaging tests (e.g., ultrasonography) are increasingly used in case of suspected GCA, the assessment of the clinical probability based on symptoms, signs and lab findings remains crucial. To standardize the estimation of clinical probability, the Southend pre-test probability score (SPTPS) has been recently developed. The SPTPS stratifies patients into low-risk, intermediate-risk and high-risk categories1,2. Retrospective studies from many centres3-6 have externally validated this SPTPS. The current study aimed to validate the SPTPS in a multicentre, prospective study.
Methods: This prospective, multicentre, observational study includes consecutive patients with suspected new-onset GCA recruited at 7 European centres participating in the HAS GCA study. SPTPS was calculated, and patients were stratified into the three risk categories: low-risk < 9, intermediate-risk 9-12 and high-risk >12. All patients underwent vascular ultrasonography (bilateral temporal arteries; common, parietal, frontal branches and axillary arteries). Vascular ultrasonography was considered positive for GCA when the intimal medial thickness is >0.42mm in common temporal, >0.29mm in Parietal, >0.34mm in frontal and >1.0mm in axillary arteries. Additional tests such as temporal artery biopsy, FDG-PET/CT, or CTA were performed at the treating physician's discretion. The final diagnosis was confirmed after 6 months of follow up.
Results: A total of 226 patients were included in the study. A diagnosis of GCA was confirmed in 83 (36.73%) patients. SPTPS was low-risk in 66 (29.2%) patients, intermediate-risk in71 (31.4%) patients and high-risk in 89 (39.4%) patients (Image). The number of patients with GCA among patients in distinct risk categories was 0 (0%) in low-risk patients, 17 (20.5%) in intermediate-risk patients and 66 (79.5%) in high-risk patients. Among the three risk categories, high diagnostic accuracy was observed for ultrasonography (table).
Conclusion: The SPTPS is a useful tool for assessing the clinical probability of GCA among patients with suspected new-onset GCA. A combination of the SPTPS and vascular ultrasonography accurately discriminated between patients with and without GCA in our prospective, multicentre study.
References:
1. Laskou F, et al. Clin Exp Rheumatol. 2019 Feb 15
2. Sebastian A, et al. RMD Open. 2020;6(3)
3. Melville et al. Rheum Adv in Practice, Volume 6, Issue 1, 2022, rkab102
4. Oshinsky et al. Arthritis Rheumatol. 2021; 73 (suppl 10)
5. Mathake et al. Arthritis Rheumatol. 2021; 73 (suppl 10)
6. Fernández-Fernández et al. RMD Open 2022 Apr;8(1):e002120


Disclosures: A. Sebastian, None; a. tomelleri, None; P. MACCHIONI, None; G. Klinowski, None; C. Salvarani, None; A. Kayani, None; M. Tariq, None; D. Prieto-Peña, UCB, Roche, Pfizer, Amgen, Janssen, AbbVie/Abbott, Novartis, Eli Lilly; E. Conticini, None; M. Khurshid, None; S. Inness, None; J. Jackson, None; K. van der Geest, Roche; B. Dasgupta, Roche, AbbVie/Abbott, sanofi.

