Back
Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes I: Diagnosis and Disease Activity (1609–1614)
1614: Females with Non-Radiographic but Not with Radiographic Axial Spondyloarthritis Are Less Likely to Achieve Inactive Disease State Than Males: Results from a Multicountry Prospective Observational Study
Sunday, November 13, 2022
5:45 PM – 5:55 PM Eastern Time
Location: Room 113
.png)
Denis Poddubnyy, MD
Charité-Universitätsmedizin Berlin
Berlin, Germany
Presenting Author(s)
Denis Poddubnyy1, Joachim Sieper2, Servet Akar3, Santiago Muñoz-Fernández4, Hildrun Haibel2, Torsten Diekhoff5, Mikhail Protopopov6, Elisabeth Altmaier7, Fabiana Ganz8 and Robert Inman9, 1Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 2Charité - Universitätsmedizin, Berlin, Berlin, Germany, 3Izmir Katip Celebi University School of Medicine, Izmir, Turkey, 4Servicio de Reumatología, Hospital Universitario Infanta Sofía, Universidad Europea, Madrid, Spain, 5Department of Radiology, Charité-Universitätsmedizin Berlin, Berlin, Germany, 6Charité Universitätsmedizin Berlin, Berlin, Germany, 7GKM Gesellschaft für Therapieforschung mbH, Munich, Germany, 8AbbVie, Inc., Luzern, Switzerland, 9University Health Network, Toronto, ON, Canada
Background/Purpose: Emerging evidence suggests that men and women experience axial spondyloarthritis (axSpA) differently. Some studies suggested that the probability of treatment response was lower in women vs men with axSpA. The objective of this analysis of PROOF was to assess the probability of achieving inactive disease/low disease activity in men vs women with recently diagnosed axSpA.
Methods: PROOF was a 5-year, global, real-world, prospective, observational study conducted in rheumatology clinical practices in 29 countries across 6 geographic regions. The study enrolled patients (pts) with recently diagnosed (within 12 months) axSpA fulfilling the ASAS classification criteria. Included patients were further classified as radiographic (r-axSpA) or non-radiographic (nr-axSpA) based on the central evaluation of radiographs of sacroiliac joints. Demographics, clinical disease characteristics, and treatment were assessed at baseline (BL) and annually thereafter. In this analysis, we included pts who had both BL and year 1 data available.
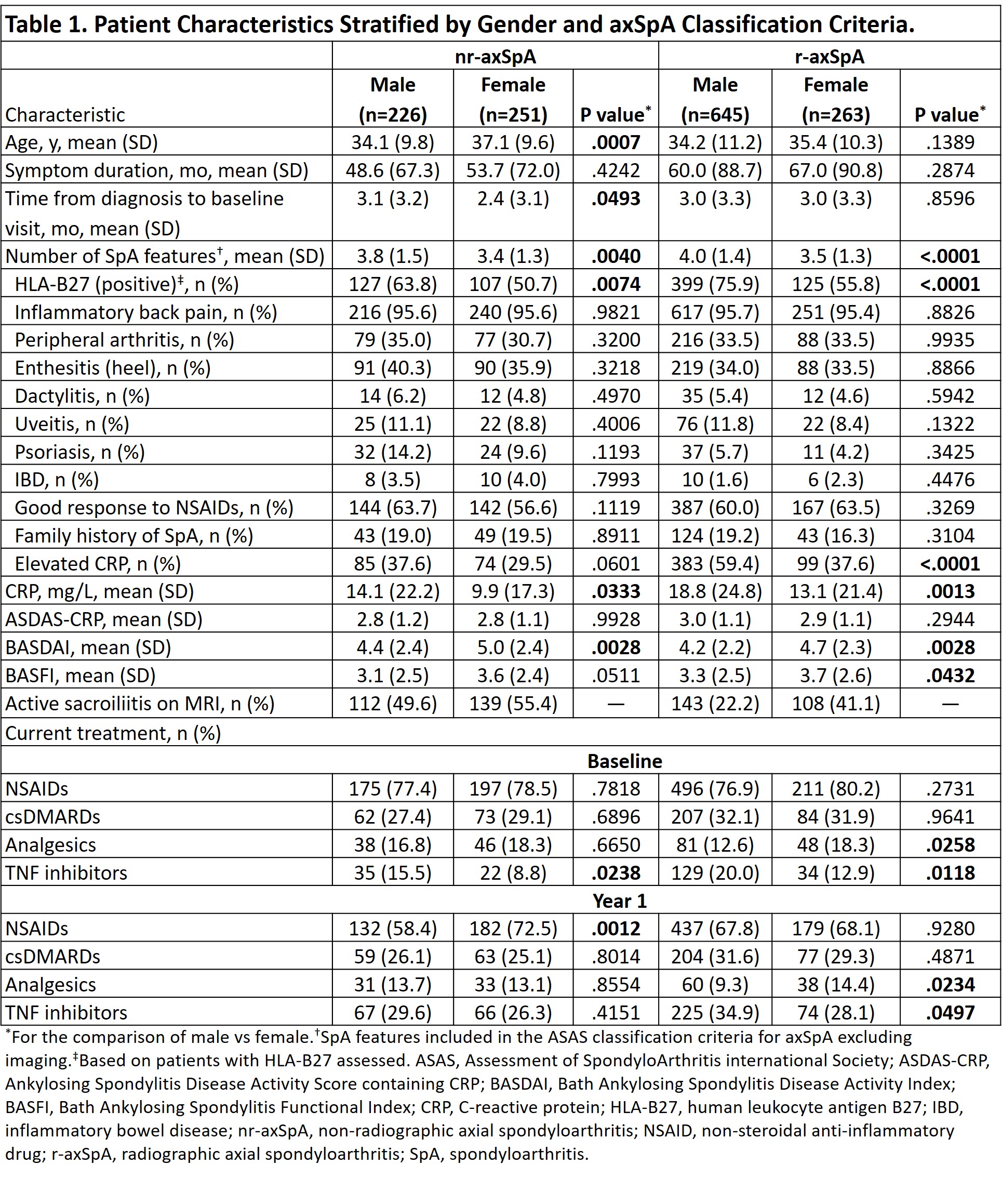
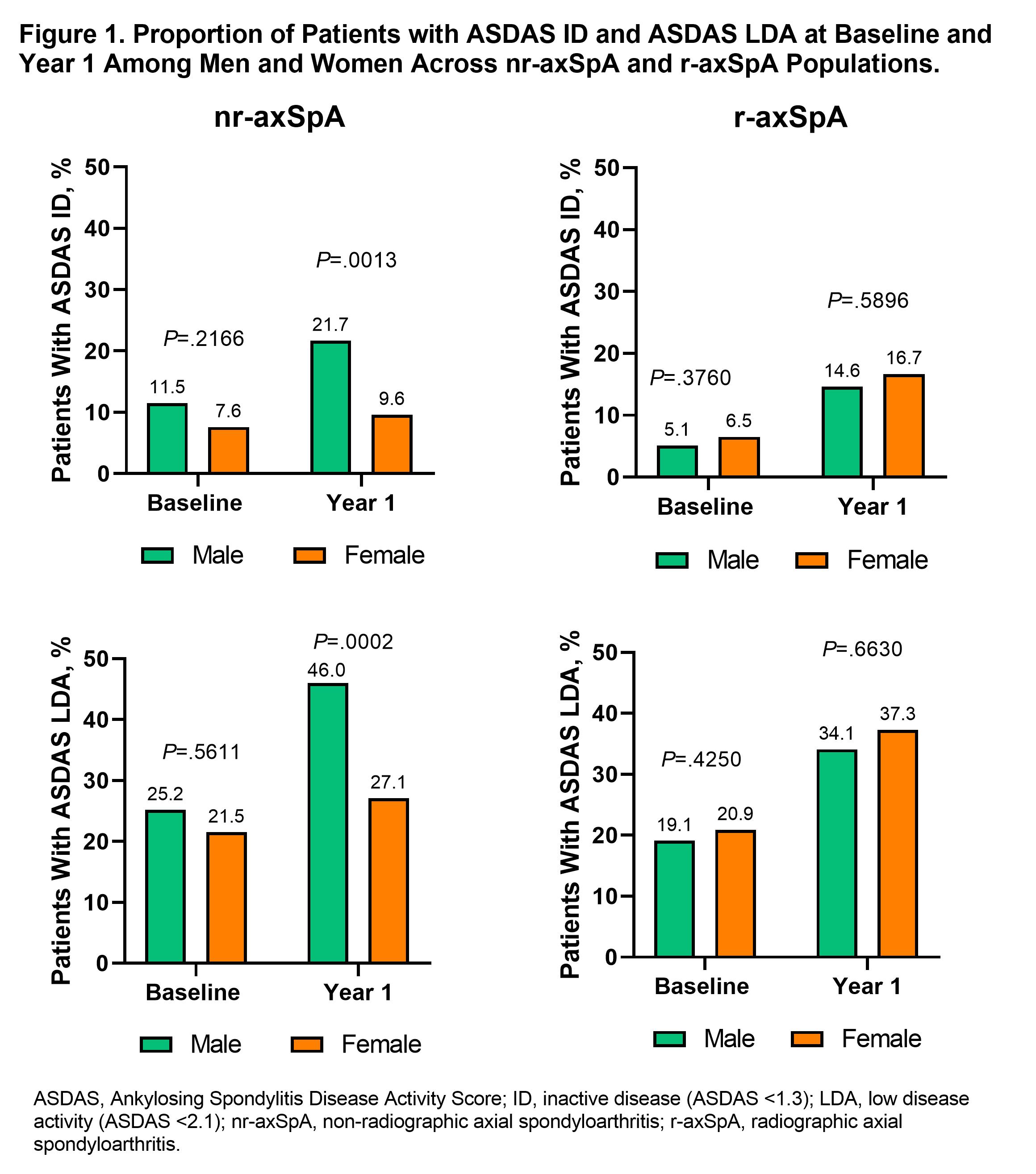
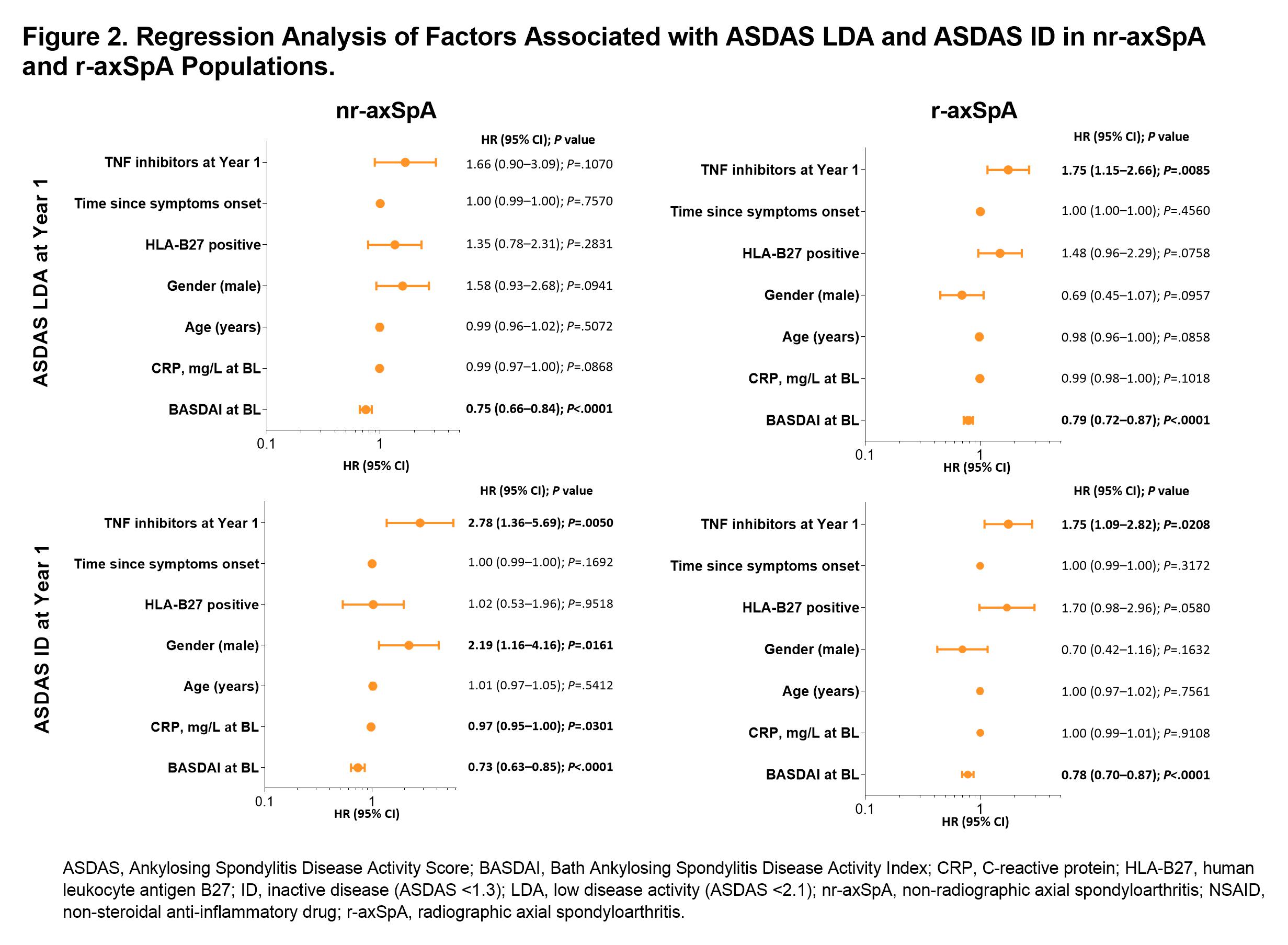
Results: Of 2633 enrolled pts, 1612 underwent central reading; of these 1385 had BL and year 1 data and were classified as having nr-axSpA (477 [34%]) or r-axSpA (908 [66%]). Among nr-axSpA pts, about half were men (n=226 [47%]); among r-axSpA pts, most were men (n=645 [71%)]). BL characteristics of the included patients stratified according to the classification status and gender are presented in Table 1. As previously reported, men were more frequently HLA-B27 positive and had higher levels of CRP than women in both nr- and r-axSpA. At BL, men had higher TNF inhibitor (TNFi) use in both nr-axSpA (15% vs 9%; P=.0238) and r-axSpA (20% vs 13%; P=.0118). At year 1, TNFi use had increased overall and remained significantly higher for men vs women with r-axSpA but not with nr-axSpA (Table 1). Disease activity generally improved from BL to year 1 for men and women in both populations; however, significantly more men vs women in the nr-axSpA population achieved low disease activity/inactive disease, while there were no differences in the r-axSpA group. (Figure 1). In the multivariable logistic regression analysis (adjusted for TNFi use and other factors), female gender was independently associated with a significantly lower odds of achieving inactive disease activity state in nr- but not in r-axSpA (Figure 2).
Conclusion: Females with nr-axSpA but not r-axSpA were less likely to achieve inactive disease state (and to a further extent, low disease activity) over time as compared with males. This effect was independent of TNFi treatment, suggesting that non-TNF-dependent mechanisms might be responsible for the presence of symptoms in females diagnosed with nr-axSpA.
Disclosures: D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB; J. Sieper, AbbVie/Abbott, Novartis, Eli Lilly, Merck/MSD, UCB, Janssen, Pfizer, Roche; S. Akar, AbbVie, Lilly, MSD, Novartis, Pfizer, UCB; S. Muñoz-Fernández, AbbVie, BMS, Galapagos, Janssen, MSD, Novartis, Pfizer, Roche, UCB; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; T. Diekhoff, Novartis, MSD, Canon MS, Lilly; M. Protopopov, None; E. Altmaier, AbbVie; F. Ganz, AbbVie; R. Inman, Novartis, Janssen, AbbVie/Abbott, Eli Lilly, Aria Pharmaceuticals, Amgen, Pfizer, Sandoz.
Background/Purpose: Emerging evidence suggests that men and women experience axial spondyloarthritis (axSpA) differently. Some studies suggested that the probability of treatment response was lower in women vs men with axSpA. The objective of this analysis of PROOF was to assess the probability of achieving inactive disease/low disease activity in men vs women with recently diagnosed axSpA.
Methods: PROOF was a 5-year, global, real-world, prospective, observational study conducted in rheumatology clinical practices in 29 countries across 6 geographic regions. The study enrolled patients (pts) with recently diagnosed (within 12 months) axSpA fulfilling the ASAS classification criteria. Included patients were further classified as radiographic (r-axSpA) or non-radiographic (nr-axSpA) based on the central evaluation of radiographs of sacroiliac joints. Demographics, clinical disease characteristics, and treatment were assessed at baseline (BL) and annually thereafter. In this analysis, we included pts who had both BL and year 1 data available.
Results: Of 2633 enrolled pts, 1612 underwent central reading; of these 1385 had BL and year 1 data and were classified as having nr-axSpA (477 [34%]) or r-axSpA (908 [66%]). Among nr-axSpA pts, about half were men (n=226 [47%]); among r-axSpA pts, most were men (n=645 [71%)]). BL characteristics of the included patients stratified according to the classification status and gender are presented in Table 1. As previously reported, men were more frequently HLA-B27 positive and had higher levels of CRP than women in both nr- and r-axSpA. At BL, men had higher TNF inhibitor (TNFi) use in both nr-axSpA (15% vs 9%; P=.0238) and r-axSpA (20% vs 13%; P=.0118). At year 1, TNFi use had increased overall and remained significantly higher for men vs women with r-axSpA but not with nr-axSpA (Table 1). Disease activity generally improved from BL to year 1 for men and women in both populations; however, significantly more men vs women in the nr-axSpA population achieved low disease activity/inactive disease, while there were no differences in the r-axSpA group. (Figure 1). In the multivariable logistic regression analysis (adjusted for TNFi use and other factors), female gender was independently associated with a significantly lower odds of achieving inactive disease activity state in nr- but not in r-axSpA (Figure 2).
Conclusion: Females with nr-axSpA but not r-axSpA were less likely to achieve inactive disease state (and to a further extent, low disease activity) over time as compared with males. This effect was independent of TNFi treatment, suggesting that non-TNF-dependent mechanisms might be responsible for the presence of symptoms in females diagnosed with nr-axSpA.



Disclosures: D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB; J. Sieper, AbbVie/Abbott, Novartis, Eli Lilly, Merck/MSD, UCB, Janssen, Pfizer, Roche; S. Akar, AbbVie, Lilly, MSD, Novartis, Pfizer, UCB; S. Muñoz-Fernández, AbbVie, BMS, Galapagos, Janssen, MSD, Novartis, Pfizer, Roche, UCB; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; T. Diekhoff, Novartis, MSD, Canon MS, Lilly; M. Protopopov, None; E. Altmaier, AbbVie; F. Ganz, AbbVie; R. Inman, Novartis, Janssen, AbbVie/Abbott, Eli Lilly, Aria Pharmaceuticals, Amgen, Pfizer, Sandoz.

