Back
Plenary Session
Crystal arthropathies
Session: Plenary III
1678: A Genome-Wide Association Analysis of 2,622,830 Individuals Reveals New Pathogenic Pathways in Gout
Monday, November 14, 2022
12:15 PM – 12:25 PM Eastern Time
Location: Exhibit Hall A
- TM
Tony Merriman, PhD
University of Alabama at Birmingham
Homewood, AL, United States
Presenting Author(s)
Tony Merriman1, Hirotaka Matsuo2, Riku Takei3, Megan Leask3, Ruth Topless1, Yuya Shirai4, Zhiqiang Li5, Murray Cadzow1, Richard Reynolds3, kenneth saag3, Tayaza Fadason6, Justin O'Sullivan6, Nicola Dalbeth6, Lisa Stamp7, Abhishek Abhishek8, Michael Doherty8, Edward Roddy9, Lennart Jacobsson10, Meliha Kapetanovic11, Mariano Andrès12, Fernando Perez-Ruiz13, Rosa Torres Jimenez14, Timothy Radstake15, Timothy Jansen16, Matthijs Janssen17, Leo Joosten18, Tania Octavia Crisan19, Tom Huizinga20, Frederic LIOTE21, Pascal Richette22, Thomas Bardin23, Tristan Pascart24, Geraldine McCarthy25, Blanka Stiburkova26, Anne Tausche27, Till Uhlig28, Veronique Vitart29, Philip Riches29, Stuart Ralston29, Thomas MacDonald30, Akiyoshi Nakayama2, Masahiro Nakatochi31, Kimiyoshi Ichida32, Tappei Takada33, Chaeyoung Lee34, Matthew Brown35, Philip Robinson36, Catherine Hill37, Hyon Choi38, Nicholas Sumpter3, Marilyn Merriman3, Amanda Phipps-Green1, Wenhua Wei1, Sally McCormick1, Olle Melander39, René Toes20, Hang-Korng Ea21, Fina Kurreeman20, Laura Helbert25, Thibaud Boutin29, Nariyoshi Shinomiya2, Linda Bradbury40, Russell Buchanan41, Susan Lester37, Malcolm Smith42, Maureen Rischmueller43, On behalf of Japan Gout Genomics Consortium (J-Gout)44, On behalf of Japan Multi-Instl Collab Cohort Study (J-MICC)45, Eli Stahl46, Jeff Miner47, Daniel Solomon48, Jing Cui48, Kathleen Giacomini49, Deanna Brackman49, Eric Jorgenson50, On behalf of 23andMe Research Team51, Suyash Shringapure51, Alexander So52, Yukinori Okada4, Changgui Li5, Yongyong Shi53 and Tanya Major1, 1University of Otago, Dunedin, New Zealand, 2National Defense Medical College, Saitama, Japan, 3University of Alabama at Birmingham, Birmingham, AL, 4Osaka University, Suita, Osaka, Japan, 5The Affiliated Hospital of Qingdao University, Qingdao, China, 6University of Auckland, Auckland, New Zealand, 7University of Otago, Christchurch, New Zealand, 8University of Nottingham, Nottingham, United Kingdom, 9Keele University, Keele, United Kingdom, 10Department of Rheumatology and Inflammation Research, Sahlgrenska Academy at Gothenburg University, Gothenburg, Sweden, 11Lund University, Department for clinical sciences Lund, section of rheumatology and Lund University Hospital Lund and Malmö, Lund, Sweden, 12Dr Balmis Alicante General University Hospital-ISABIAL, Alicante, Spain, 13University of the Basque Country, Barakaldo, Spain, 14La Paz University Hospital, Madrid, Spain, 15University Medical College Uthrecht, Utrecht, Netherlands, 16VieCuri Medical Centre, Venlo, Netherlands, 17Rijnstate Hospital, Bennekom, Netherlands, 18Radboudumc, Nijmegen, Netherlands, 19University of Medicine and Pharmacy \"Iuliu Hatieganu\" Cluj-Napoca, Cluj-Napoca, Romania, 20Leiden University Medical Center, Leiden, Netherlands, 21University of Paris, Paris, France, 22Department of Rheumatology, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, Paris, France, 23Hôpital Lariboisiere, Paris, France, 24Lille Catholic University, Lille, France, 25Mater Misericordiae University Hospital, Dublin, Ireland, 26Institute of Rheumatology, Prague, Czech Republic, 27University Clinic 'Carl Gustav Carus' at the Technical University, Dresden, Germany, 28Diakonhjemmet Hospital, Oslo, Norway, 29University of Edinburgh, Edinburgh, Scotland, United Kingdom, 30University of Dundee, Dundee, United Kingdom, 31Nagoya University Hospital, Nagoya, Japan, 32Tokyo University of Pharmacy and Life Sciences, Hachioji, Japan, 33University of Tokyo Hospital, Tokyo, Japan, 34Soongsil University, Seoul, Republic of Korea, 35Genomics England, London, United Kingdom, 36University of Queensland, Brisbane, Australia, 37The Queen Elizabeth Hospital, Adelaide, Australia, 38Massachusetts General Hospital, Lexington, MA, 39Lund University, Malmö, Sweden, 40Gold Coast University Hospital, Brisbane, Australia, 41Austin Health, Heidelberg, Australia, 42Flinders Medical Centre, Adelaide, Australia, 43RheumatologySA, Adelaide, Australia, 44Japan Gout Genomics Consortium (J-Gout), Saitama, Japan, 45Japan Multi-Institutional Collaborative Cohort Study (J-MICC), Nagoya, Japan, 46Regeneron, New York, NY, 47ViscientBio, San Diego, CA, 48Brigham and Women's Hospital, Boston, MA, 49University of California San Francisco, San Francisco, CA, 50Kaiser Permanente, San Francisco, CA, 5123andMe, Inc., Sunnyvale, CA, 52University of Lausanne, Lausanne, Switzerland, 53Shanghai Jiao Tong University, Shanghai, China
Background/Purpose: Genome-wide association studies (GWAS) in gout have been relatively small (≤13,179 people with gout) and have provided little insight into the progression from hyperuricemia to gout. We present a gout GWAS that includes 120,282 people with gout.
Methods: Participants were from 13 cohorts and four ancestral groups (African 3,052 gout/77,891 non-gout; East Asian 10,729/82,807; European 100,661/2,106,003; Latina 5,840/235,847). Ancestry-specific GWAS summary statistics were meta-analysed to form the trans-ancestral GWAS. Candidate causal genes were identified by co-localization with expression quantitative trait loci (eQTL) using the Gene and Tissue Expression dataset and/or having a candidate missense causal variant and/or by MAGMA gene set analysis. IL-1β response QTL were identified using the 500FG cohort. Pathway analysis was done using KEGG and drug repositioning analysis was done using Genome for Repositioning Drugs.
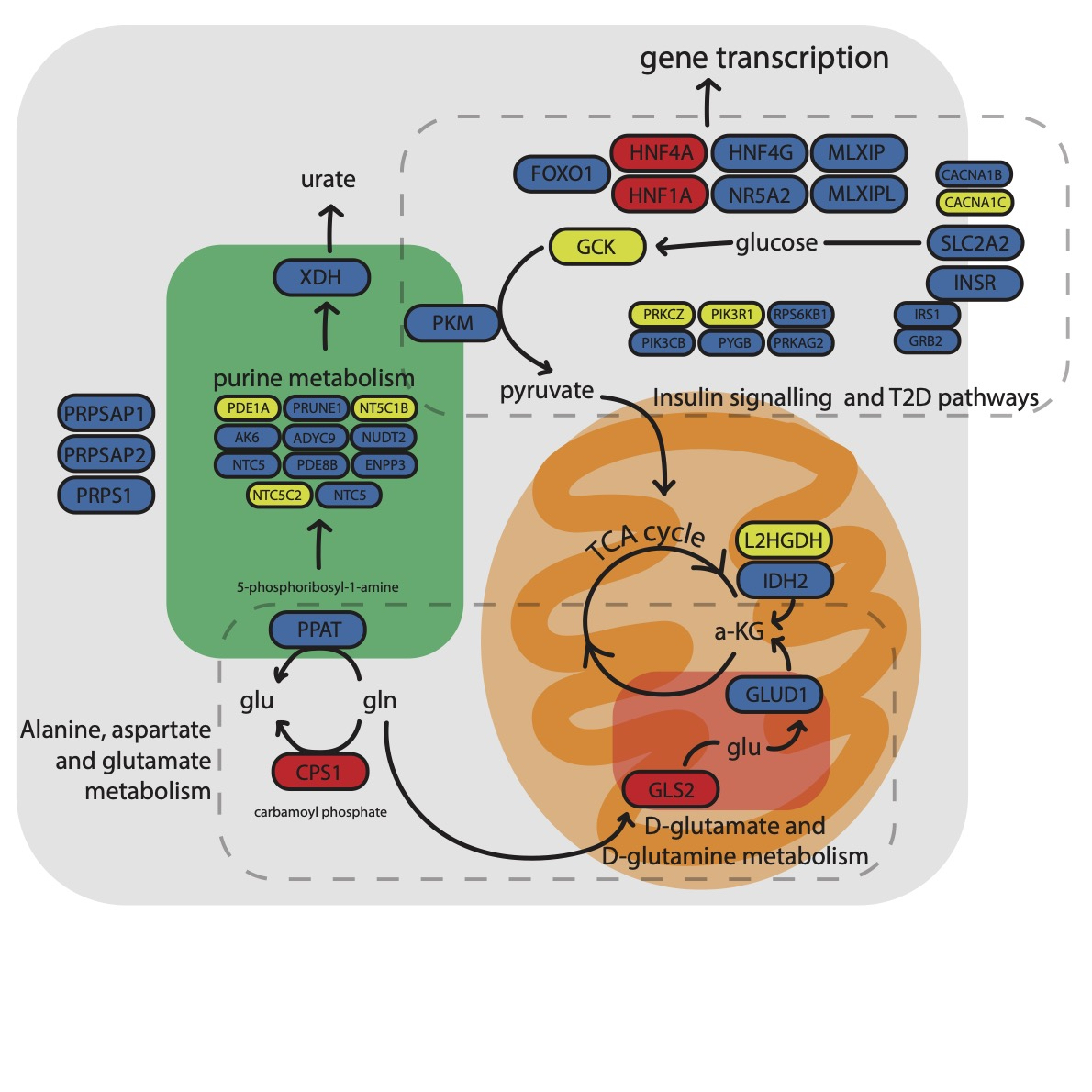
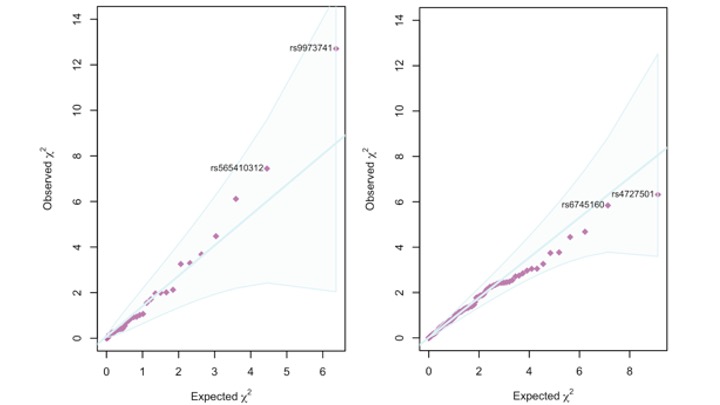
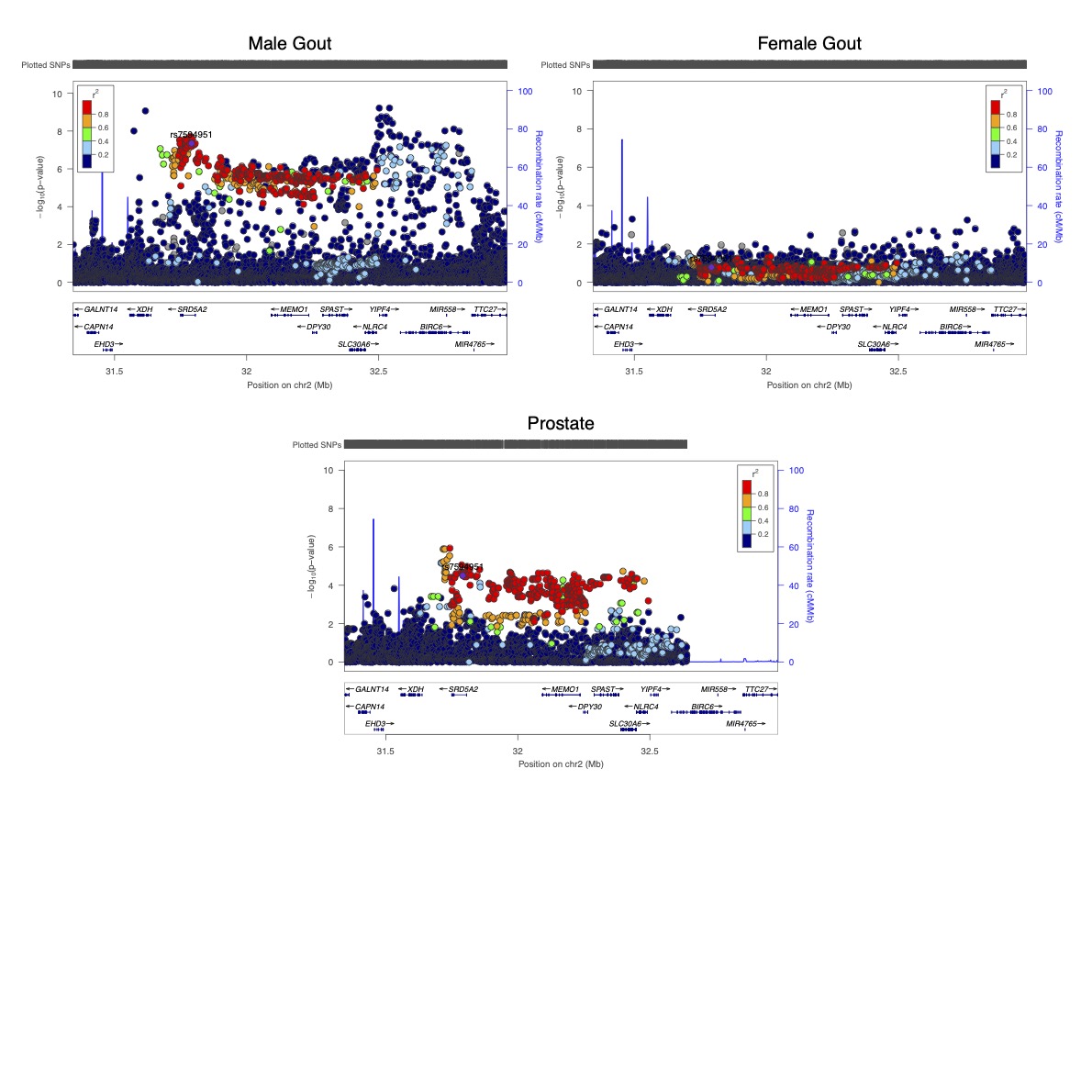
Results: There were 339 gout-associated loci encompassing 515 independently-associated variants. 123 loci did not overlap with any previously reported in urate or gout. Co-localization of GWAS and eQTL signals identified 1,657 cis- and 267 trans-eQTLs for 252 of the 339 loci. Some co-localized eQTL genes regulate NLRP3 inflammasome activation and activity (e.g. TMEM176B, SCAP, INSIG2 SREBF-AS1, FADS2, NLRC3). Co-localized eQTLs also included TET2, EZH2, IDH2, and RUNX1, genes mutated in Clonal Hematopoiesis of Indeterminate Potential (CHIP). Enriched molecular pathways using candidate genes identified purine metabolism, amino acid metabolism and insulin signalling pathways (Figure 1). Genetic variants present in the 500FG dataset and not previously associated with urate (n=93, possibly involved in inflammation in gout) had amplification of signal that associated with IL-1b response to monosodium urate (MSU) crystal and LPS co-stimulation in peripheral blood mononuclear cells (Figure 2). However those associated with urate (n=317) did not exhibit amplified signal (Figure 2). The gout GWAS signal at the top-associated variant of the 93 variants not associated with urate (rs9973741) co-localized with genetic control of production of IL-1b upon stimulation (the gout risk allele associated with increased IL-1β level), and also co-localized with genetic control (eQTL) of IL1RN and IL-38 expression, implicating these genes as causal at this locus. An association signal at Xanthine Dehydrogenase was restricted to males and co-localized with XDH expression (eQTL) only in the prostate (Figure 3). Gout-associated loci represented drug repurposing possibilities from neoplasm, blood biochemistry, and metabolic disorder treatment categories.
Conclusion: We implicate multiple new causal genes and pathways in gout. In addition to genes that regulate urate, NLRP3 inflammasome activity and autophagy, this work has identified the CHIP pathway, that includes epigenome modification proteins, in gout pathogenesis. Other pathways identified involve insulin signalling and purine and glutamate metabolism. Uniquely regulated production of urate by the prostate potentially represents a novel risk factor for gout in men.
Disclosures: T. Merriman, None; H. Matsuo, None; R. Takei, None; M. Leask, None; R. Topless, None; Y. Shirai, None; Z. Li, None; M. Cadzow, None; R. Reynolds, None; k. saag, Horizon Therapeutics, SOBI, Shanton, AbbVie/Abbott; T. Fadason, None; J. O'Sullivan, None; N. Dalbeth, AstraZeneca, Dyve Biosciences, Horizon, Selecta, JW Pharmaceutical Corporation, PK Med, PTC Therapeutics, Protalix; L. Stamp, None; A. Abhishek, AstraZeneca, Pfizer, Oxford immunotec, Inflazome, NGMBiopharmaceuticals, Uptodate, Cadilla pharmaceuticals; M. Doherty, None; E. Roddy, None; L. Jacobsson, Novartis, Eli Lilly, Janssen; M. Kapetanovic, None; M. Andrès, Menarini, Grunenthal; F. Perez-Ruiz, None; R. Torres Jimenez, None; T. Radstake, None; T. Jansen, None; M. Janssen, None; L. Joosten, None; T. Crisan, None; T. Huizinga, None; F. LIOTE, None; P. Richette, AbbVie, Amgen, Biogen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sanofi-Aventis, UCB; T. Bardin, None; T. Pascart, None; G. McCarthy, None; B. Stiburkova, None; A. Tausche, None; T. Uhlig, None; V. Vitart, None; P. Riches, None; S. Ralston, None; T. MacDonald, Menarini; A. Nakayama, None; M. Nakatochi, None; K. Ichida, None; T. Takada, None; C. Lee, None; M. Brown, UCB Pharma, Pfizer, Clementia, Ipsen, Incyte, Regeneron, Grey Wolf Therapeutics, Xinthera, Novartis; P. Robinson, None; C. Hill, None; H. Choi, Horizon, Allena, LG, Protalix; N. Sumpter, None; M. Merriman, None; A. Phipps-Green, None; W. Wei, None; S. McCormick, None; O. Melander, None; R. Toes, None; H. Ea, None; F. Kurreeman, None; L. Helbert, None; T. Boutin, None; N. Shinomiya, None; L. Bradbury, Novartis; R. Buchanan, None; S. Lester, None; M. Smith, None; M. Rischmueller, None; O. Japan Gout Genomics Consortium (J-Gout), None; O. Japan Multi-Instl Collab Cohort Study (J-MICC), None; E. Stahl, None; J. Miner, None; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; J. Cui, None; K. Giacomini, None; D. Brackman, None; E. Jorgenson, None; O. 23andMe Research Team, 23andMe, Inc.; S. Shringapure, None; A. So, None; Y. Okada, None; C. Li, None; Y. Shi, None; T. Major, None.
Background/Purpose: Genome-wide association studies (GWAS) in gout have been relatively small (≤13,179 people with gout) and have provided little insight into the progression from hyperuricemia to gout. We present a gout GWAS that includes 120,282 people with gout.
Methods: Participants were from 13 cohorts and four ancestral groups (African 3,052 gout/77,891 non-gout; East Asian 10,729/82,807; European 100,661/2,106,003; Latina 5,840/235,847). Ancestry-specific GWAS summary statistics were meta-analysed to form the trans-ancestral GWAS. Candidate causal genes were identified by co-localization with expression quantitative trait loci (eQTL) using the Gene and Tissue Expression dataset and/or having a candidate missense causal variant and/or by MAGMA gene set analysis. IL-1β response QTL were identified using the 500FG cohort. Pathway analysis was done using KEGG and drug repositioning analysis was done using Genome for Repositioning Drugs.
Results: There were 339 gout-associated loci encompassing 515 independently-associated variants. 123 loci did not overlap with any previously reported in urate or gout. Co-localization of GWAS and eQTL signals identified 1,657 cis- and 267 trans-eQTLs for 252 of the 339 loci. Some co-localized eQTL genes regulate NLRP3 inflammasome activation and activity (e.g. TMEM176B, SCAP, INSIG2 SREBF-AS1, FADS2, NLRC3). Co-localized eQTLs also included TET2, EZH2, IDH2, and RUNX1, genes mutated in Clonal Hematopoiesis of Indeterminate Potential (CHIP). Enriched molecular pathways using candidate genes identified purine metabolism, amino acid metabolism and insulin signalling pathways (Figure 1). Genetic variants present in the 500FG dataset and not previously associated with urate (n=93, possibly involved in inflammation in gout) had amplification of signal that associated with IL-1b response to monosodium urate (MSU) crystal and LPS co-stimulation in peripheral blood mononuclear cells (Figure 2). However those associated with urate (n=317) did not exhibit amplified signal (Figure 2). The gout GWAS signal at the top-associated variant of the 93 variants not associated with urate (rs9973741) co-localized with genetic control of production of IL-1b upon stimulation (the gout risk allele associated with increased IL-1β level), and also co-localized with genetic control (eQTL) of IL1RN and IL-38 expression, implicating these genes as causal at this locus. An association signal at Xanthine Dehydrogenase was restricted to males and co-localized with XDH expression (eQTL) only in the prostate (Figure 3). Gout-associated loci represented drug repurposing possibilities from neoplasm, blood biochemistry, and metabolic disorder treatment categories.
Conclusion: We implicate multiple new causal genes and pathways in gout. In addition to genes that regulate urate, NLRP3 inflammasome activity and autophagy, this work has identified the CHIP pathway, that includes epigenome modification proteins, in gout pathogenesis. Other pathways identified involve insulin signalling and purine and glutamate metabolism. Uniquely regulated production of urate by the prostate potentially represents a novel risk factor for gout in men.

Figure 1. Molecular pathways causally implicated in gout pathogenesis.

Figure 2. Q-Q plots of association of non-urate-associated variants (n=93, left panel) and urate-associated variants (n=317, right panel) with IL-1b level after co-stimulation of PBMCs by MSU crystals and LPS.

Figure 3. LocusZoom plots of gout association signal at the XDH locus in males (top left) and females (top right) from the European GWAS. Genetic control of XDH expression, specific to the prostate, is in the bottom plot. XDH is the fourth gene from the left in each plot. Individual genetic variants are represented as dots; genome position is on the x-axis and strength of evidence for association on the y-axis; genetic variants in strong linkage disequilibrium with the lead variant at the prostate-specific signal (rs7594591) are in red.
Disclosures: T. Merriman, None; H. Matsuo, None; R. Takei, None; M. Leask, None; R. Topless, None; Y. Shirai, None; Z. Li, None; M. Cadzow, None; R. Reynolds, None; k. saag, Horizon Therapeutics, SOBI, Shanton, AbbVie/Abbott; T. Fadason, None; J. O'Sullivan, None; N. Dalbeth, AstraZeneca, Dyve Biosciences, Horizon, Selecta, JW Pharmaceutical Corporation, PK Med, PTC Therapeutics, Protalix; L. Stamp, None; A. Abhishek, AstraZeneca, Pfizer, Oxford immunotec, Inflazome, NGMBiopharmaceuticals, Uptodate, Cadilla pharmaceuticals; M. Doherty, None; E. Roddy, None; L. Jacobsson, Novartis, Eli Lilly, Janssen; M. Kapetanovic, None; M. Andrès, Menarini, Grunenthal; F. Perez-Ruiz, None; R. Torres Jimenez, None; T. Radstake, None; T. Jansen, None; M. Janssen, None; L. Joosten, None; T. Crisan, None; T. Huizinga, None; F. LIOTE, None; P. Richette, AbbVie, Amgen, Biogen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sanofi-Aventis, UCB; T. Bardin, None; T. Pascart, None; G. McCarthy, None; B. Stiburkova, None; A. Tausche, None; T. Uhlig, None; V. Vitart, None; P. Riches, None; S. Ralston, None; T. MacDonald, Menarini; A. Nakayama, None; M. Nakatochi, None; K. Ichida, None; T. Takada, None; C. Lee, None; M. Brown, UCB Pharma, Pfizer, Clementia, Ipsen, Incyte, Regeneron, Grey Wolf Therapeutics, Xinthera, Novartis; P. Robinson, None; C. Hill, None; H. Choi, Horizon, Allena, LG, Protalix; N. Sumpter, None; M. Merriman, None; A. Phipps-Green, None; W. Wei, None; S. McCormick, None; O. Melander, None; R. Toes, None; H. Ea, None; F. Kurreeman, None; L. Helbert, None; T. Boutin, None; N. Shinomiya, None; L. Bradbury, Novartis; R. Buchanan, None; S. Lester, None; M. Smith, None; M. Rischmueller, None; O. Japan Gout Genomics Consortium (J-Gout), None; O. Japan Multi-Instl Collab Cohort Study (J-MICC), None; E. Stahl, None; J. Miner, None; D. Solomon, AbbVie/Abbott, Amgen, moderna, CorEvitas; J. Cui, None; K. Giacomini, None; D. Brackman, None; E. Jorgenson, None; O. 23andMe Research Team, 23andMe, Inc.; S. Shringapure, None; A. So, None; Y. Okada, None; C. Li, None; Y. Shi, None; T. Major, None.

