Back
Poster Session D
Epidemiology, health policy and outcomes
Session: (1750–1786) Epidemiology and Public Health Poster III
1767: Epidemiology of Eosinophilic Granulomatosis with Polyangiitis (EGPA) and Hypereosinophilic Syndrome (HES) in Germany: A Claims Database Study
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- BH
BERNHARD HELLMICH, MD
Medius Kliniken
Kirchheim unter Teck, Germany
Abstract Poster Presenter(s)
Bernhard Hellmich1, Konstantin Neukirch2, Marco Lukas2, Martin Wernitz2, Dominik Beier3 and Dennis Häckl4, 1Department of Internal Medicine, Rheumatology and Immunology, Medius Kliniken, University of Tübingen, Kirchheim Teck, Germany, 2GlaxoSmithKline GmbH & Co. KG, Munich, Germany, 3InGef - Institute for Applied Health Research Berlin GmbH, Berlin, Germany, 4WIG2 GmbH, Leipzig, Germany
Background/Purpose: EGPA and HES are rare multisystemic diseases associated with eosinophilia. Robust data on the epidemiology and treatment of HES and EGPA are scarce. The aim of this study was to describe the epidemiology and treatment of HES and EGPA in Germany by analyzing population-based health claims data.
Methods: The study was conducted with the InGef research database, which contains anonymized data from approximately 4 million insured persons of 60 German statutory health insurances, representative to the age- and sex-structure of Germany. The study was designed as a cross-sectional study with an observation period from 2014-2019. An EGPA case was defined as a diagnosis according to ICD-10-GM code M30.1 (Polyarteritis with lung involvement [Churg-Strauss]), either as a main- or secondary inpatient diagnosis, or as two verified outpatient diagnoses in different quarters within the respective study year. In case of verified outpatient diagnoses, a prescription of corticosteroids, cyclophosphamide, ciclosporin, rituximab, leflunomide, mycophenolic acid, methotrexate or azathioprine had to be made in at least one of the quarters in which EGPA was diagnosed. In the same approach, a HES case was defined as a diagnosis according to ICD-10-GM code D72.1 (Eosinophilia) or D47.5 (Chronic eosinophilic leukaemia [HES]) and in case of outpatient diagnoses, a prescription of corticosteroids, immunosuppressive agents (azathioprine, interferon alfa, peginterferon alfa, ciclosporin), hydroxycarbamide or imatinib had to be made. Data from the representative sample were extrapolated to the German population.
Results: Projected to the total German population, prevalence of EGPA and HES in 2019 were 3.89 and 10.44, while incidence proportions were 0.75 patients and 3.95 per 100,000 population, respectively. Both prevalence and incidence increased during the observation period. EGPA prevalence and incidence were higher in females (56% and 64%), while HES prevalence and incidence proportions were comparable between male and female populations (51% and 49% female). New EGPA cases were almost exclusively found in patients over 18 years of age. HES incidence proportion was mostly higher in the younger age groups (e.g., for 2018, patients < 12 years and patients between 12 and < 18 years vs. patients ≥18 years: 4.80 and 4.31 cases vs. 3.38 cases per 100,000 population under risk). The majority of both EGPA and HES patients was treated with corticosteroids and about a quarter of EGPA patients with azathioprine. Asthma was among the most frequent comorbidity (EGPA: 70.70%; HES: 54.74% of patients).
Conclusion: To our knowledge, this is the largest study on the epidemiology of patients with EGPA and HES in Germany reported so far. Although both diseases are rare, prevalence increased continuously in the years 2015-2019. EGPA and HES were diagnosed more frequently than it was estimated by previous self-reporting registry-based studies. The findings indicate that in the past epidemiological data of EGPA and HES may have been underestimated but may also reflect improved diagnostic methods and disease recognition.
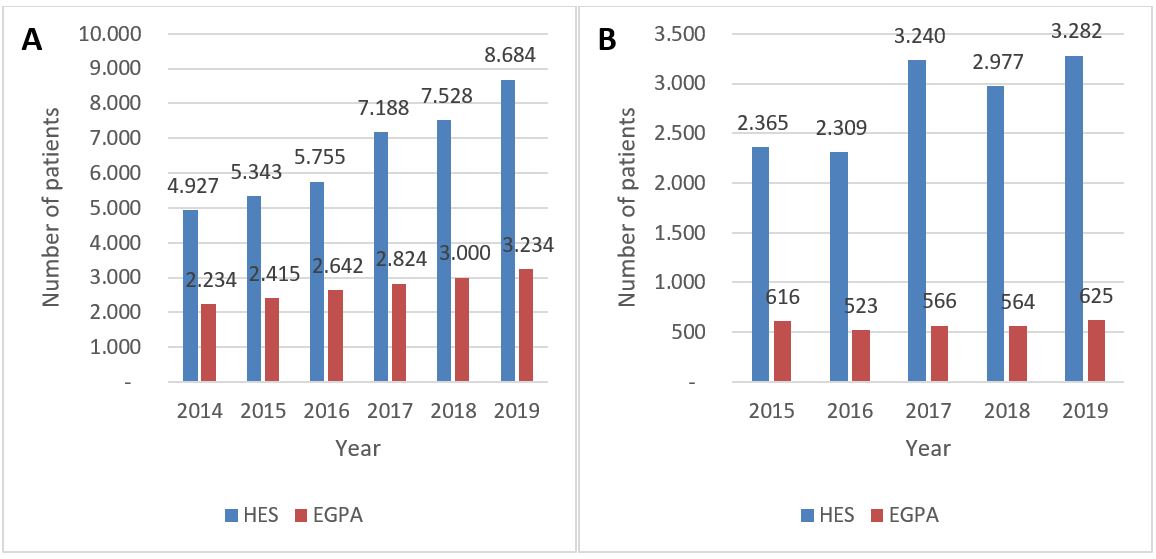 Figure 1. Prevalence and incidence of HES and EGPA. (A) Number of prevalent EGPA and HES cases projected to total German population. (B) Number of incident EGPA and HES cases projected to total German population.
Figure 1. Prevalence and incidence of HES and EGPA. (A) Number of prevalent EGPA and HES cases projected to total German population. (B) Number of incident EGPA and HES cases projected to total German population.
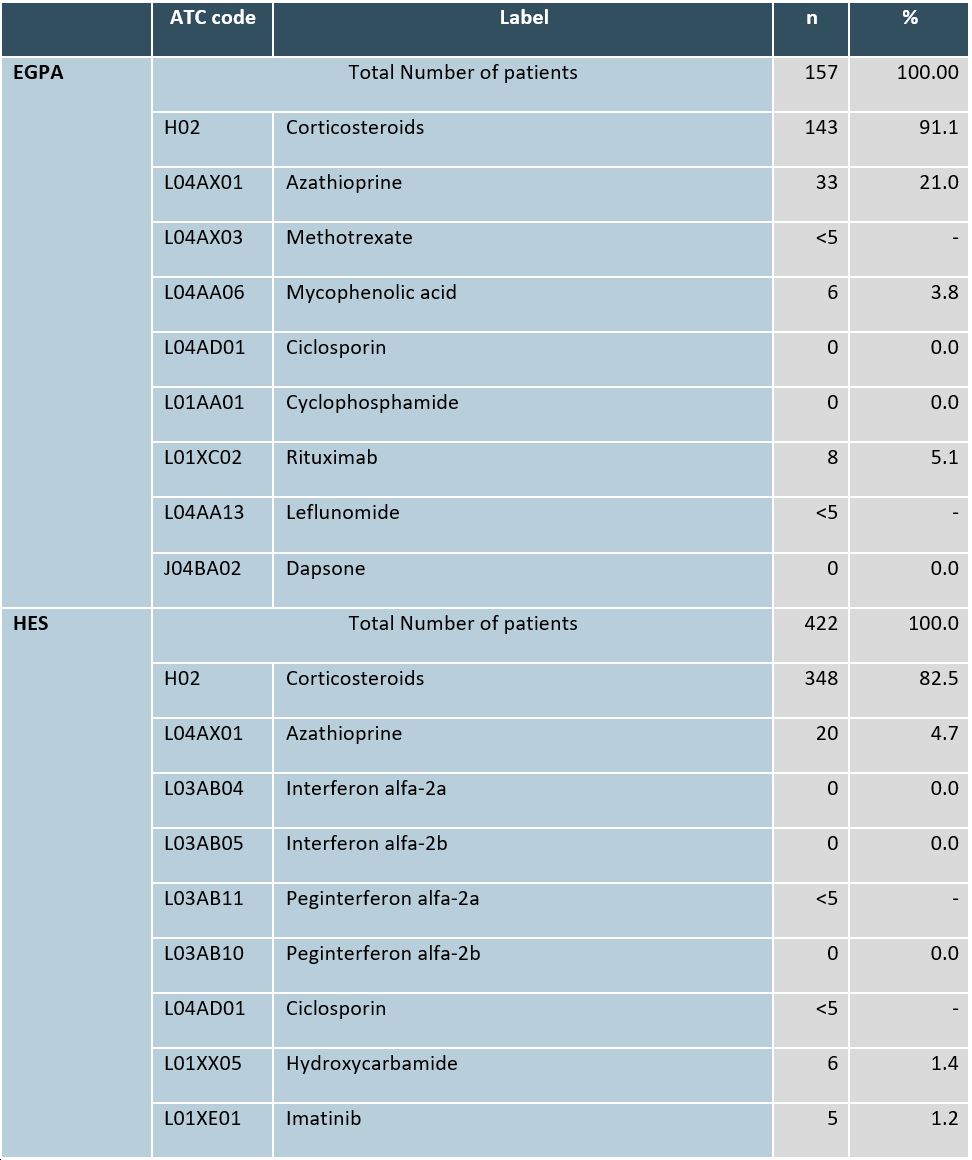 Table 1: Prescribed medications for EGPA and HES patients in 2019
Table 1: Prescribed medications for EGPA and HES patients in 2019
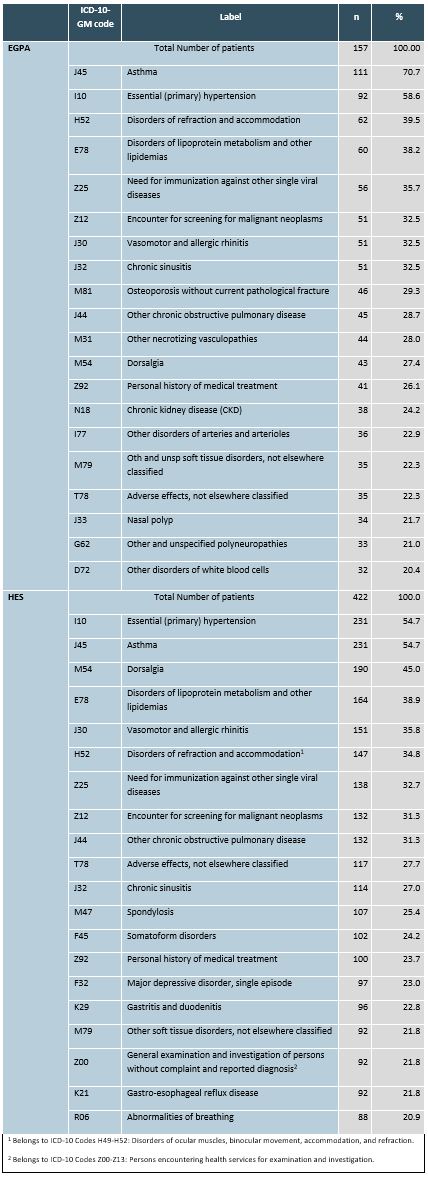 Table 2: 20 most common comorbid diagnoses according to ICD-10-GM in EGPA and HES patients in 2019
Table 2: 20 most common comorbid diagnoses according to ICD-10-GM in EGPA and HES patients in 2019
Disclosures: B. Hellmich, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Chugai, GlaxoSmithKline (GSK), InflaRx, Merck/MSD, Novartis, Roche, Vifor, AbbVie/Abbott, Boehringer-Ingelheim, Janssen, Pfizer, Phadia; K. Neukirch, GlaxoSmithKline (GSK); M. Lukas, GlaxoSmithKline (GSK); M. Wernitz, GlaxoSmithKline (GSK); D. Beier, GlaxoSmithKline (GSK); D. Häckl, GlaxoSmithKline (GSK).
Background/Purpose: EGPA and HES are rare multisystemic diseases associated with eosinophilia. Robust data on the epidemiology and treatment of HES and EGPA are scarce. The aim of this study was to describe the epidemiology and treatment of HES and EGPA in Germany by analyzing population-based health claims data.
Methods: The study was conducted with the InGef research database, which contains anonymized data from approximately 4 million insured persons of 60 German statutory health insurances, representative to the age- and sex-structure of Germany. The study was designed as a cross-sectional study with an observation period from 2014-2019. An EGPA case was defined as a diagnosis according to ICD-10-GM code M30.1 (Polyarteritis with lung involvement [Churg-Strauss]), either as a main- or secondary inpatient diagnosis, or as two verified outpatient diagnoses in different quarters within the respective study year. In case of verified outpatient diagnoses, a prescription of corticosteroids, cyclophosphamide, ciclosporin, rituximab, leflunomide, mycophenolic acid, methotrexate or azathioprine had to be made in at least one of the quarters in which EGPA was diagnosed. In the same approach, a HES case was defined as a diagnosis according to ICD-10-GM code D72.1 (Eosinophilia) or D47.5 (Chronic eosinophilic leukaemia [HES]) and in case of outpatient diagnoses, a prescription of corticosteroids, immunosuppressive agents (azathioprine, interferon alfa, peginterferon alfa, ciclosporin), hydroxycarbamide or imatinib had to be made. Data from the representative sample were extrapolated to the German population.
Results: Projected to the total German population, prevalence of EGPA and HES in 2019 were 3.89 and 10.44, while incidence proportions were 0.75 patients and 3.95 per 100,000 population, respectively. Both prevalence and incidence increased during the observation period. EGPA prevalence and incidence were higher in females (56% and 64%), while HES prevalence and incidence proportions were comparable between male and female populations (51% and 49% female). New EGPA cases were almost exclusively found in patients over 18 years of age. HES incidence proportion was mostly higher in the younger age groups (e.g., for 2018, patients < 12 years and patients between 12 and < 18 years vs. patients ≥18 years: 4.80 and 4.31 cases vs. 3.38 cases per 100,000 population under risk). The majority of both EGPA and HES patients was treated with corticosteroids and about a quarter of EGPA patients with azathioprine. Asthma was among the most frequent comorbidity (EGPA: 70.70%; HES: 54.74% of patients).
Conclusion: To our knowledge, this is the largest study on the epidemiology of patients with EGPA and HES in Germany reported so far. Although both diseases are rare, prevalence increased continuously in the years 2015-2019. EGPA and HES were diagnosed more frequently than it was estimated by previous self-reporting registry-based studies. The findings indicate that in the past epidemiological data of EGPA and HES may have been underestimated but may also reflect improved diagnostic methods and disease recognition.
 Figure 1. Prevalence and incidence of HES and EGPA. (A) Number of prevalent EGPA and HES cases projected to total German population. (B) Number of incident EGPA and HES cases projected to total German population.
Figure 1. Prevalence and incidence of HES and EGPA. (A) Number of prevalent EGPA and HES cases projected to total German population. (B) Number of incident EGPA and HES cases projected to total German population. Table 1: Prescribed medications for EGPA and HES patients in 2019
Table 1: Prescribed medications for EGPA and HES patients in 2019 Table 2: 20 most common comorbid diagnoses according to ICD-10-GM in EGPA and HES patients in 2019
Table 2: 20 most common comorbid diagnoses according to ICD-10-GM in EGPA and HES patients in 2019Disclosures: B. Hellmich, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Chugai, GlaxoSmithKline (GSK), InflaRx, Merck/MSD, Novartis, Roche, Vifor, AbbVie/Abbott, Boehringer-Ingelheim, Janssen, Pfizer, Phadia; K. Neukirch, GlaxoSmithKline (GSK); M. Lukas, GlaxoSmithKline (GSK); M. Wernitz, GlaxoSmithKline (GSK); D. Beier, GlaxoSmithKline (GSK); D. Häckl, GlaxoSmithKline (GSK).

