Back
Poster Session D
Sjögren's syndrome
Session: (2017–2051) Sjögren's Syndrome – Basic and Clinical Science Poster
2025: Natural History of Sjögren's Disease from the National Institutes of Health Cohort
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- BL
Brandon Law, MD
Brandon Law
Baltimore, MD, United States
Abstract Poster Presenter(s)
Brandon Law1, Margaret Beach2, Eileen Pelayo2, Ilias Alevizos2, Zohreh Khavandgar2, Blake Warner2 and Alan Baer1, 1Johns Hopkins University School of Medicine, Baltimore, MD, 2National Institutes of Health, Bethesda, MD
Background/Purpose: The objectives of this study were to assess the natural history of Sjögren's disease (SjD) over an interval reaching up to 32 years among participants of the U.S. National Institutes of Health (NIH) cohort.
Methods: Research participants consented and enrolled on salivary gland dysfunction-focused protocols at the NIH Sjögren's Disease Clinic between 1984 and 2020 were included in this analysis. Assessments varied according to protocol, but generally included baseline comprehensive serologic/rheumatologic, oral, and ocular assessments including objective measures of salivary function (e.g., sialometry), tear flow, minor salivary gland biopsy (MSGB), and laboratory testing. Sialometric measurements included unstimulated whole salivary (UWS) flow rate and total unstimulated salivary flow rate, which is the sum of parotid and submandibular/sublingual unstimulated salivary flow. The "percent unchanged" was defined as the percentage of participants with concordant test results at baseline and follow-up. For continuous variables, binary indicators using established cutoff values were used. Percentages were summarized with exact binomial 95% confidence intervals.
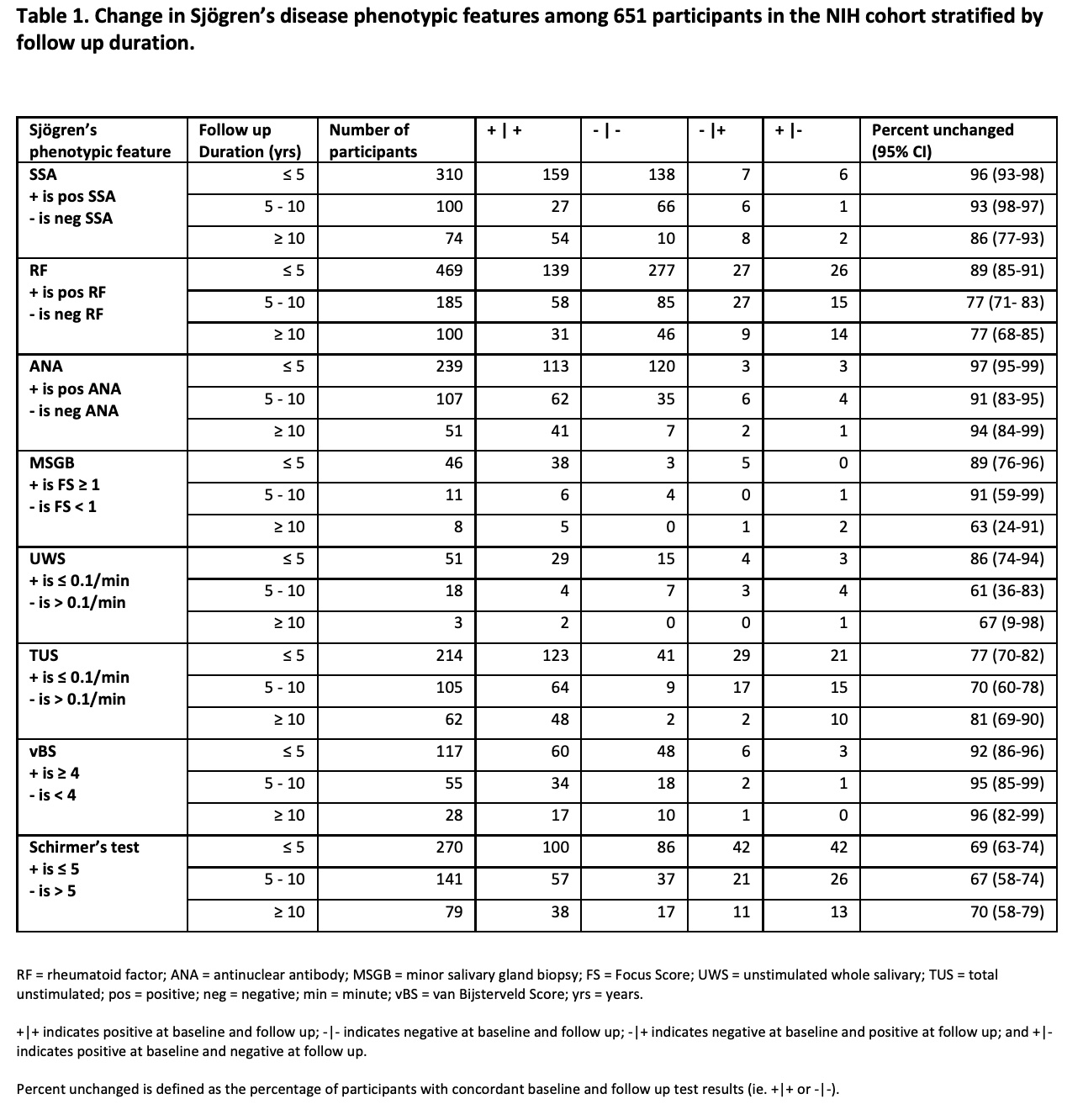
Results: Among 2,813 individuals evaluated, 1,198 had baseline evaluations that enabled classification into one of three subgroups: 1) 779 with SjD, 2) 127 with SjD coexistent with another systemic rheumatic disease, and 3) 292 with sufficient data to exclude SjD (non-SjD) with available baseline features. The majority of participants in the primary SjD subgroup were White (73%) with a mean age of 51 (SD=13) years. 73% had anti-SSA antibodies, 59% rheumatoid factor (RF), and 96% focus score ≥1 on MSGB. Follow up was available for 651 participants and stratified by follow up durations of ≤ 5 years, between 5-10 years, and ≥ 10 years. As summarized in Table 1, the percent unchanged of follow up durations of ≤ 5 years ranged from 69% for Schirmer's test (n=270) to 97% for ANA (n=239). The percent unchanged remained high for participants with ≥ 10 years of follow up with 70% for Schirmer's test (n=79) and 94% for ANA (n=51). The percent unchanged for both UWS flow and MSGB for durations of > 5 years were limited by small sample size. Among participants with a baseline Focus Score (FS) ≥1 with serial MSGB (n=52), 94% still had this at follow up and included 11 participants with a repeat positive FS at follow up durations of ≥ 5 years. Of the participants categorized as SjD at baseline, 94% again met criteria at follow up. Among participants who did not meet criteria for SjD at baseline, 7% (n=6) progressed to meet criteria at follow up. Conversely, 6% (n=7) of participants who met criteria for SjD at baseline did not meet criteria at follow up.
Conclusion: This appreciably large, single center cohort with longitudinal follow up provides rich detail from which to understand the natural history of SjD. A key takeaway of this study was the relative stability in the serologic, oral/sialometric, and ocular measurements over the follow-up interval. These data are critical to future clinical trials for designing appropriate endpoints.
Disclosures: B. Law, None; M. Beach, None; E. Pelayo, None; I. Alevizos, None; Z. Khavandgar, None; B. Warner, Pfizer, Astellas Bio; A. Baer, None.
Background/Purpose: The objectives of this study were to assess the natural history of Sjögren's disease (SjD) over an interval reaching up to 32 years among participants of the U.S. National Institutes of Health (NIH) cohort.
Methods: Research participants consented and enrolled on salivary gland dysfunction-focused protocols at the NIH Sjögren's Disease Clinic between 1984 and 2020 were included in this analysis. Assessments varied according to protocol, but generally included baseline comprehensive serologic/rheumatologic, oral, and ocular assessments including objective measures of salivary function (e.g., sialometry), tear flow, minor salivary gland biopsy (MSGB), and laboratory testing. Sialometric measurements included unstimulated whole salivary (UWS) flow rate and total unstimulated salivary flow rate, which is the sum of parotid and submandibular/sublingual unstimulated salivary flow. The "percent unchanged" was defined as the percentage of participants with concordant test results at baseline and follow-up. For continuous variables, binary indicators using established cutoff values were used. Percentages were summarized with exact binomial 95% confidence intervals.
Results: Among 2,813 individuals evaluated, 1,198 had baseline evaluations that enabled classification into one of three subgroups: 1) 779 with SjD, 2) 127 with SjD coexistent with another systemic rheumatic disease, and 3) 292 with sufficient data to exclude SjD (non-SjD) with available baseline features. The majority of participants in the primary SjD subgroup were White (73%) with a mean age of 51 (SD=13) years. 73% had anti-SSA antibodies, 59% rheumatoid factor (RF), and 96% focus score ≥1 on MSGB. Follow up was available for 651 participants and stratified by follow up durations of ≤ 5 years, between 5-10 years, and ≥ 10 years. As summarized in Table 1, the percent unchanged of follow up durations of ≤ 5 years ranged from 69% for Schirmer's test (n=270) to 97% for ANA (n=239). The percent unchanged remained high for participants with ≥ 10 years of follow up with 70% for Schirmer's test (n=79) and 94% for ANA (n=51). The percent unchanged for both UWS flow and MSGB for durations of > 5 years were limited by small sample size. Among participants with a baseline Focus Score (FS) ≥1 with serial MSGB (n=52), 94% still had this at follow up and included 11 participants with a repeat positive FS at follow up durations of ≥ 5 years. Of the participants categorized as SjD at baseline, 94% again met criteria at follow up. Among participants who did not meet criteria for SjD at baseline, 7% (n=6) progressed to meet criteria at follow up. Conversely, 6% (n=7) of participants who met criteria for SjD at baseline did not meet criteria at follow up.
Conclusion: This appreciably large, single center cohort with longitudinal follow up provides rich detail from which to understand the natural history of SjD. A key takeaway of this study was the relative stability in the serologic, oral/sialometric, and ocular measurements over the follow-up interval. These data are critical to future clinical trials for designing appropriate endpoints.

Disclosures: B. Law, None; M. Beach, None; E. Pelayo, None; I. Alevizos, None; Z. Khavandgar, None; B. Warner, Pfizer, Astellas Bio; A. Baer, None.

