Back
Poster Session D
Crystal arthropathies
Session: (1787–1829) Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
1807: Reduction in Monosodium Urate Crystal Deposit Volume During the MIRROR RCT Trial in Patients Treated with Pegloticase Plus Methotrexate Co-therapy: A Serial Dual-Energy Computed Tomography (DECT) Analysis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AK
Ada Kumar, MD
Medical Director, Medical Affairs and Outcomes Research
Horizon Therapeutics
Deerfield, IL, United States
Abstract Poster Presenter(s)
Nicola Dalbeth1, John Botson2, kenneth saag3, Ada Kumar4, Lissa Padnick-Silver5, Brian LaMoreaux5 and Fabio Becce6, 1University of Auckland, Auckland, New Zealand, 2Orthopedic Physicians Alaska, Anchorage, AK, 3University of Alabama at Birmingham, Birmingham, AL, 4Horizon Therapeutics, Deerfield, IL, 5Horizon Therapeutics plc, Deerfield, IL, 6Lausanne University Hospital (CHUV), Lausanne, Switzerland
Background/Purpose: Dual-energy computed tomography (DECT) can reliably visualize and quantify monosodium urate (MSU) crystal deposits in gout patients.1 Two MIRROR open-label trial (pegloticase + oral methotrexate [MTX] co-therapy) participants underwent serial DECT imaging, both showing a rapid, progressive decrease in MSU deposition volume during therapy.2 The MIRROR randomized controlled trial (MIRROR RCT) showed increased urate-lowering response rate to pegloticase when co-administered with MTX (71.0% vs. 38.5% with monotherapy during Month 6)3 and included a larger number of participants who underwent serial DECT imaging during therapy. Here, we report serial DECT MSU deposition findings from MIRROR RCT.
Methods: MIRROR RCT (NCT03635957) participants were randomized 2:1 to receive pegloticase (8 mg biweekly infusions) plus blinded MTX (15 mg/wk) or placebo (PBO). At DECT-capable sites, serial images were obtained using a standardized acquisition protocol at specified points during the 52-wk treatment period (Day 1 [pegloticase infusion 1]; Wks 14, 24, 52). Bilateral hand/wrist, elbow, foot/ankle, and knee images were obtained, post-processed using default settings, and interpreted by an independent central reader who was blinded to treatment group and response. MSU deposition volume was assessed in each scan. Patients with baseline and Wk 52 images were included in analyses. Joints with a baseline MSU volume < 0.5 cm3 were excluded to avoid large contributions of potential DECT artifacts.4
Results: 6 MTX and 2 PBO patients were included in analyses. 5 MTX patients were treatment responders during Month 6, receiving 52 wks of pegloticase + MTX. The remaining patient discontinued pegloticase + MTX (nonresponder) at Wk 6, beginning allopurinol (maintained sUA < 6 mg/dL thru Wk 52). 1 PBO patient was a responder during Month 6, receiving 42 wks of pegloticase + PBO. The remaining patient discontinued pegloticase + PBO (nonresponder) at Wk 6, beginning allopurinol (maintained sUA < 6 mg/dL thru Wk 52). MSU volume progressively and markedly decreased during therapy in both treatment groups (Wk 52, MTX: -93.8% ± 8.8% across 9 imaging regions of 6 patients, PBO: -95.7% ± 3.1% across 4 imaging regions of 2 patients). The 2 patients who received 6 wks of pegloticase + MTX/PBO co-therapy had MSU volume changes of -72.3% (MTX) and -97.1% (PBO) at Wk 52. MSU volume reductions were comparable in different regions of the same patient and occurred similarly with urate-lowering in both treatment arms.
Conclusion: DECT imaging showed that pegloticase + MTX/PBO co-therapy led to rapid debulking of MSU deposition across multiple joints, with a mean urate volume reduction of 95% over 52 wks. Meaningful debulking was also observed in 2 nonresponders (6 wks of pegloticase) who immediately began allopurinol with pegloticase discontinuation. These findings provide further evidence of MSU burden reduction across joints with pegloticase-induced urate lowering.
References
1. Choi HK et al. Ann Rheum Dis 2012, 71:1466-71
2. Dalbeth N et al. Rheumatology (Oxford) 2022 [Epub ahead of print]
3. Botson et al. Ann Rheum Dis 2022;81(suppl 1):112
4. Coupal TM et al. AJR Am J Roentgenol 2016;206:119-28
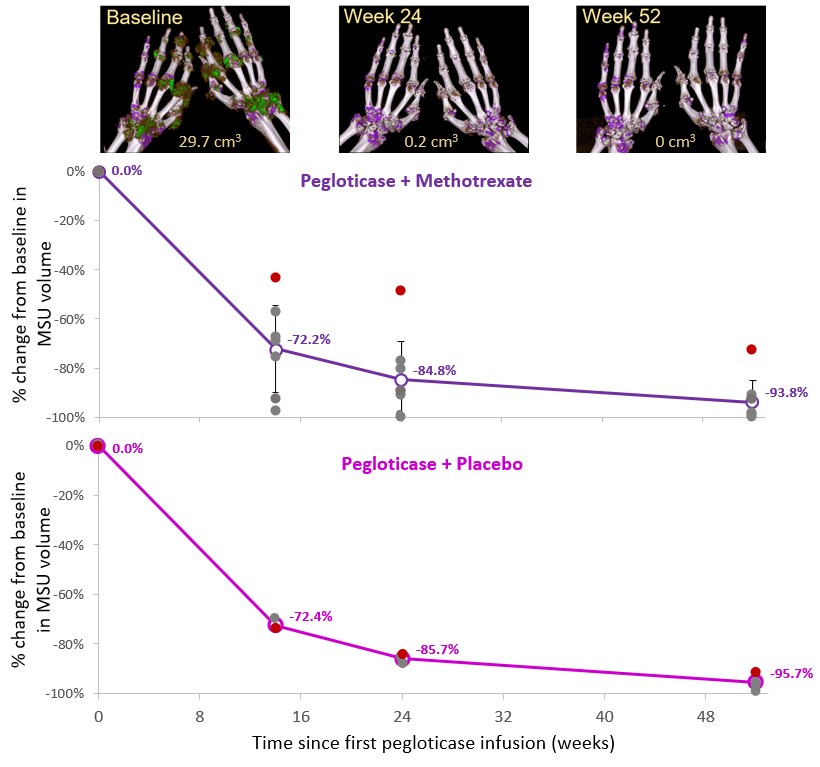 Figure. Monosodium urate (MSU) deposition volume change over time, as measured by serial DECT imaging, in patients undergoing pegloticase therapy with methotrexate (Top) or placebo (Bottom) as co-therapy. Mean changes are shown (open circles), along with individual scan changes (closed circles). One patient in each treatment group received only 6 weeks of therapy (red circles), beginning allopurinol at Week 6 (MTX: 300 mg/day titrated up to 600 mg/day at Week 12; PBO: 600 mg/day). Error bars represent standard deviation. Bilateral hand/wrist volume-rendered DECT images showing MSU deposition (green) and scan MSU volume are shown for one patient in the methotrexate arm.
Figure. Monosodium urate (MSU) deposition volume change over time, as measured by serial DECT imaging, in patients undergoing pegloticase therapy with methotrexate (Top) or placebo (Bottom) as co-therapy. Mean changes are shown (open circles), along with individual scan changes (closed circles). One patient in each treatment group received only 6 weeks of therapy (red circles), beginning allopurinol at Week 6 (MTX: 300 mg/day titrated up to 600 mg/day at Week 12; PBO: 600 mg/day). Error bars represent standard deviation. Bilateral hand/wrist volume-rendered DECT images showing MSU deposition (green) and scan MSU volume are shown for one patient in the methotrexate arm.
Disclosures: N. Dalbeth, AstraZeneca, Dyve Biosciences, Horizon, Selecta, JW Pharmaceutical Corporation, PK Med, PTC Therapeutics, Protalix; J. Botson, Horizon Therapeutics, Abbvie, Amgen, Allena, Radius Health, Aurinia, Chemocentryx, Lilly, Novartis; k. saag, Horizon Therapeutics, SOBI, Shanton, AbbVie/Abbott; A. Kumar, Horizon Therapeutics; L. Padnick-Silver, Horizon Therapeutics; B. LaMoreaux, Horizon Therapeutics; F. Becce, Horizon Therapeutics, Siemens Healthineers.
Background/Purpose: Dual-energy computed tomography (DECT) can reliably visualize and quantify monosodium urate (MSU) crystal deposits in gout patients.1 Two MIRROR open-label trial (pegloticase + oral methotrexate [MTX] co-therapy) participants underwent serial DECT imaging, both showing a rapid, progressive decrease in MSU deposition volume during therapy.2 The MIRROR randomized controlled trial (MIRROR RCT) showed increased urate-lowering response rate to pegloticase when co-administered with MTX (71.0% vs. 38.5% with monotherapy during Month 6)3 and included a larger number of participants who underwent serial DECT imaging during therapy. Here, we report serial DECT MSU deposition findings from MIRROR RCT.
Methods: MIRROR RCT (NCT03635957) participants were randomized 2:1 to receive pegloticase (8 mg biweekly infusions) plus blinded MTX (15 mg/wk) or placebo (PBO). At DECT-capable sites, serial images were obtained using a standardized acquisition protocol at specified points during the 52-wk treatment period (Day 1 [pegloticase infusion 1]; Wks 14, 24, 52). Bilateral hand/wrist, elbow, foot/ankle, and knee images were obtained, post-processed using default settings, and interpreted by an independent central reader who was blinded to treatment group and response. MSU deposition volume was assessed in each scan. Patients with baseline and Wk 52 images were included in analyses. Joints with a baseline MSU volume < 0.5 cm3 were excluded to avoid large contributions of potential DECT artifacts.4
Results: 6 MTX and 2 PBO patients were included in analyses. 5 MTX patients were treatment responders during Month 6, receiving 52 wks of pegloticase + MTX. The remaining patient discontinued pegloticase + MTX (nonresponder) at Wk 6, beginning allopurinol (maintained sUA < 6 mg/dL thru Wk 52). 1 PBO patient was a responder during Month 6, receiving 42 wks of pegloticase + PBO. The remaining patient discontinued pegloticase + PBO (nonresponder) at Wk 6, beginning allopurinol (maintained sUA < 6 mg/dL thru Wk 52). MSU volume progressively and markedly decreased during therapy in both treatment groups (Wk 52, MTX: -93.8% ± 8.8% across 9 imaging regions of 6 patients, PBO: -95.7% ± 3.1% across 4 imaging regions of 2 patients). The 2 patients who received 6 wks of pegloticase + MTX/PBO co-therapy had MSU volume changes of -72.3% (MTX) and -97.1% (PBO) at Wk 52. MSU volume reductions were comparable in different regions of the same patient and occurred similarly with urate-lowering in both treatment arms.
Conclusion: DECT imaging showed that pegloticase + MTX/PBO co-therapy led to rapid debulking of MSU deposition across multiple joints, with a mean urate volume reduction of 95% over 52 wks. Meaningful debulking was also observed in 2 nonresponders (6 wks of pegloticase) who immediately began allopurinol with pegloticase discontinuation. These findings provide further evidence of MSU burden reduction across joints with pegloticase-induced urate lowering.
References
1. Choi HK et al. Ann Rheum Dis 2012, 71:1466-71
2. Dalbeth N et al. Rheumatology (Oxford) 2022 [Epub ahead of print]
3. Botson et al. Ann Rheum Dis 2022;81(suppl 1):112
4. Coupal TM et al. AJR Am J Roentgenol 2016;206:119-28
 Figure. Monosodium urate (MSU) deposition volume change over time, as measured by serial DECT imaging, in patients undergoing pegloticase therapy with methotrexate (Top) or placebo (Bottom) as co-therapy. Mean changes are shown (open circles), along with individual scan changes (closed circles). One patient in each treatment group received only 6 weeks of therapy (red circles), beginning allopurinol at Week 6 (MTX: 300 mg/day titrated up to 600 mg/day at Week 12; PBO: 600 mg/day). Error bars represent standard deviation. Bilateral hand/wrist volume-rendered DECT images showing MSU deposition (green) and scan MSU volume are shown for one patient in the methotrexate arm.
Figure. Monosodium urate (MSU) deposition volume change over time, as measured by serial DECT imaging, in patients undergoing pegloticase therapy with methotrexate (Top) or placebo (Bottom) as co-therapy. Mean changes are shown (open circles), along with individual scan changes (closed circles). One patient in each treatment group received only 6 weeks of therapy (red circles), beginning allopurinol at Week 6 (MTX: 300 mg/day titrated up to 600 mg/day at Week 12; PBO: 600 mg/day). Error bars represent standard deviation. Bilateral hand/wrist volume-rendered DECT images showing MSU deposition (green) and scan MSU volume are shown for one patient in the methotrexate arm.Disclosures: N. Dalbeth, AstraZeneca, Dyve Biosciences, Horizon, Selecta, JW Pharmaceutical Corporation, PK Med, PTC Therapeutics, Protalix; J. Botson, Horizon Therapeutics, Abbvie, Amgen, Allena, Radius Health, Aurinia, Chemocentryx, Lilly, Novartis; k. saag, Horizon Therapeutics, SOBI, Shanton, AbbVie/Abbott; A. Kumar, Horizon Therapeutics; L. Padnick-Silver, Horizon Therapeutics; B. LaMoreaux, Horizon Therapeutics; F. Becce, Horizon Therapeutics, Siemens Healthineers.

