Back
Poster Session D
Sjögren's syndrome
Session: (2017–2051) Sjögren's Syndrome – Basic and Clinical Science Poster
2045: Does Ancestry Influence Primary Sjögren’s Syndrome Phenotype or Severity?
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MB
Maxime Beydon, MD
Université Paris Cité
Paris, France
Abstract Poster Presenter(s)
Maxime Beydon1, Marie Dulin2, Raphaèle Seror3, Frederic Desmoulins2, Xavier Mariette4 and Gaetane Nocturne5, 1Université Paris Cité, Paris, France, 2Kremlin Bicêtre Hospital - APHP, Paris, France, 3University Hospital Paris-Saclay, Le Kremlin Bicêtre, France, 4Paris-Saclay University, Rueil Malmaison, Ile-de-France, France, 5APHP, Le Kremlin Bicêtre, France
Background/Purpose: It is well established that in systemic lupus erythematous (SLE), disease burden is higher in patients from African ancestry (AA) than in Caucasian patients. Although primary Sjögren's syndrome (pSS) and SLE vary by their clinical presentation, they share common pathogenic mechanisms. Our objectives were to compare demographic and biological parameters as well as disease activity and therapeutic management between patients of African Ancestry (AA) and Caucasians.
Methods: We conducted a retrospective case-control study. Ethnicity was classified retrospectively asking the patient or relatives, if necessary. We included all pSS patients referred in a single national referral center for Sjögren's disease since 1990 of AA including sub-Saharan and Afro-Caribbean origins. Each AA pSS patient was matched to two Caucasians patients from the same center based on follow-up duration. Patients were excluded if they met diagnostic criteria for SLE, rheumatoid arthritis or any other connective tissue disease. We calculated a cumulative ESSDAI (cumESSDAI) score defined as the sum of each category maximum score during follow-up. Characteristics of patients were compared between patients of African Ancestry (AA) and Caucasians. Circulating B-cell activating factor (BAFF) levels and presence of BAFF-var genotype were compared between patients of African and non-African ancestry from a separate pSS cohort.
Results: We included 48 patients of AA matched to 96 Caucasians with a median follow-up of 5 years [IQR 2-10]. Patients of AA were younger with a median age at diagnosis of 40 [IQR 28–48] vs. 53 [IQR 38–60] (p < 0.001).
Anti-SSA antibodies were more frequently positive in AA patients (83% vs. 67%, p = 0.06) as well as anti-Sm (8% vs. 0%, p = 0.01) and anti-RNP (17% vs. 4%, p = 0.02). Mean serum titers of gammaglobulins was markedly higher in patients of AA (18.8g/L [IQR 15-24] vs. 12.6g/L [IQR 9.7-16.2], p < 0.001) as was any occurrence of cryoglobulinemia during follow-up (10% vs. 2%, p = 0.041. Median cumESSDAI score was higher in AA patients (9.5 [IQR 4-19] vs 4.0 [IQR 2-8] p < 0.001) with a higher prevalence of arthritis, myositis, interstitial lung disease and lymphadenopathy. CumESSDAI constitutional domain was more frequently increased in AA patients. Lymphomas occurred in five patients in both groups (10% vs 5.2% p = 0.3). Full details are presented in table 1.
Patients of AA were more often prescribed immunosuppressors, particularly Rituximab, without overall statistical significance.
We subsequently compared serum BAFF levels in 41 AA patients and 369 non-AA patients. Median BAFF levels was 1559pg/mL (IQR 1186-2032) in AA patients and 1311pg/mL (IQR 1057-1825) in non-AA patients (p = 0.02) (Figure 1). BAFF-var genotype was significantly associated with BAFF-levels (p = 0.002) however it was not significantly more prevalent in AA patients than in non-AA patients (5% vs 8.5%, p = 0.75).
Conclusion: Patients of AA seemed to have a distinct phenotype of pSS compared to Caucasian, with earlier disease onset, higher gammaglobulin titers, more frequent anti-SSA positivity and higher systemic disease activity alongside with higher circulating BAFF levels, which might not be explained by the presence of BAFF-var polymorphism.
.jpg) Table 1: Patients characteristics according to ancestry
Table 1: Patients characteristics according to ancestry
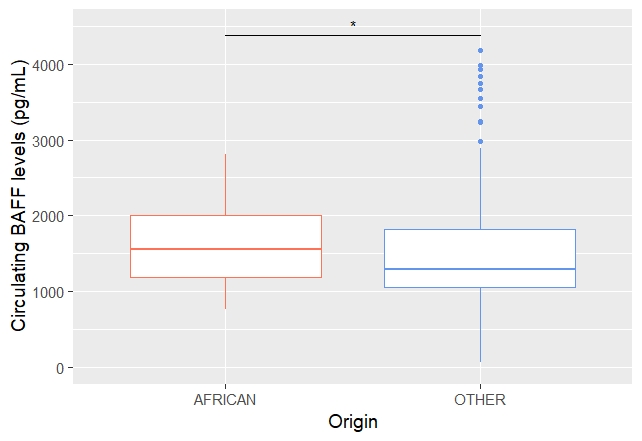 Figure 1: Circulating BAFF levels According to Ancenstry
Figure 1: Circulating BAFF levels According to Ancenstry
Disclosures: M. Beydon, None; M. Dulin, None; R. Seror, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Janssen, Novartis, Amgen; F. Desmoulins, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen.
Background/Purpose: It is well established that in systemic lupus erythematous (SLE), disease burden is higher in patients from African ancestry (AA) than in Caucasian patients. Although primary Sjögren's syndrome (pSS) and SLE vary by their clinical presentation, they share common pathogenic mechanisms. Our objectives were to compare demographic and biological parameters as well as disease activity and therapeutic management between patients of African Ancestry (AA) and Caucasians.
Methods: We conducted a retrospective case-control study. Ethnicity was classified retrospectively asking the patient or relatives, if necessary. We included all pSS patients referred in a single national referral center for Sjögren's disease since 1990 of AA including sub-Saharan and Afro-Caribbean origins. Each AA pSS patient was matched to two Caucasians patients from the same center based on follow-up duration. Patients were excluded if they met diagnostic criteria for SLE, rheumatoid arthritis or any other connective tissue disease. We calculated a cumulative ESSDAI (cumESSDAI) score defined as the sum of each category maximum score during follow-up. Characteristics of patients were compared between patients of African Ancestry (AA) and Caucasians. Circulating B-cell activating factor (BAFF) levels and presence of BAFF-var genotype were compared between patients of African and non-African ancestry from a separate pSS cohort.
Results: We included 48 patients of AA matched to 96 Caucasians with a median follow-up of 5 years [IQR 2-10]. Patients of AA were younger with a median age at diagnosis of 40 [IQR 28–48] vs. 53 [IQR 38–60] (p < 0.001).
Anti-SSA antibodies were more frequently positive in AA patients (83% vs. 67%, p = 0.06) as well as anti-Sm (8% vs. 0%, p = 0.01) and anti-RNP (17% vs. 4%, p = 0.02). Mean serum titers of gammaglobulins was markedly higher in patients of AA (18.8g/L [IQR 15-24] vs. 12.6g/L [IQR 9.7-16.2], p < 0.001) as was any occurrence of cryoglobulinemia during follow-up (10% vs. 2%, p = 0.041. Median cumESSDAI score was higher in AA patients (9.5 [IQR 4-19] vs 4.0 [IQR 2-8] p < 0.001) with a higher prevalence of arthritis, myositis, interstitial lung disease and lymphadenopathy. CumESSDAI constitutional domain was more frequently increased in AA patients. Lymphomas occurred in five patients in both groups (10% vs 5.2% p = 0.3). Full details are presented in table 1.
Patients of AA were more often prescribed immunosuppressors, particularly Rituximab, without overall statistical significance.
We subsequently compared serum BAFF levels in 41 AA patients and 369 non-AA patients. Median BAFF levels was 1559pg/mL (IQR 1186-2032) in AA patients and 1311pg/mL (IQR 1057-1825) in non-AA patients (p = 0.02) (Figure 1). BAFF-var genotype was significantly associated with BAFF-levels (p = 0.002) however it was not significantly more prevalent in AA patients than in non-AA patients (5% vs 8.5%, p = 0.75).
Conclusion: Patients of AA seemed to have a distinct phenotype of pSS compared to Caucasian, with earlier disease onset, higher gammaglobulin titers, more frequent anti-SSA positivity and higher systemic disease activity alongside with higher circulating BAFF levels, which might not be explained by the presence of BAFF-var polymorphism.
.jpg) Table 1: Patients characteristics according to ancestry
Table 1: Patients characteristics according to ancestry Figure 1: Circulating BAFF levels According to Ancenstry
Figure 1: Circulating BAFF levels According to AncenstryDisclosures: M. Beydon, None; M. Dulin, None; R. Seror, GlaxoSmithKlein(GSK), Boehringer-Ingelheim, Janssen, Novartis, Amgen; F. Desmoulins, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; G. Nocturne, Pfizer, Novartis, Eli Lilly, Amgen.

