Back
Plenary Session
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: Plenary I (0001–0005)
0002: Cancer Screening Recommendations for Patients with Idiopathic Inflammatory Myopathy
Saturday, November 12, 2022
12:00 PM – 12:10 PM Eastern Time
Location: Exhibit Hall A

Alexander Oldroyd, PhD, MSc, MBChB, MRCP
University of Manchester
Manchester, United Kingdom
Presenting Author(s)
Alexander Oldroyd1, Jeffrey Callen2, Hector Chinoy3, Lorinda Chung4, David Fiorentino5, Patrick Gordon6, Pedro Machado7, Neil McHugh8, Albert Selva O’Callaghan9, Jens Schmidt10, Sarah Tansley8, Ruth Ann Vleugels11, Victoria Werth12 and Rohit Aggarwal13, 1University of Manchester, Manchester, United Kingdom, 2University of Louisville, Louisville, KY, 3The University of Manchester, Sale, United Kingdom, 4Stanford University, Palo Alto, 5Stanford University, Stanford, CA, 6King’s College Hospital NHS Foundation Trust, London, United Kingdom, 7University College London, London, United Kingdom, 8University of Bath, Bath, United Kingdom, 9Hospital Universitari Vall d'Hebron, Barcelona, Spain, 10University Medical Centre Göttingen, Göttingen, Germany, 11Harvard Medical School, Boston, MA, 12Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, 13Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA
Background/Purpose: Adult-onset idiopathic inflammatory myopathy (IIM) is associated with increased cancer risk (lung, ovarian, colorectal, lymphoma, breast, and naso-pharyngeal among the most common) within the three years prior to or following IIM onset. Evidence and consensus-based recommendations for IIM-associated cancer screening could potentially improve outcomes.
Methods: The International Myositis Assessment and Clinical Studies Group (IMACS) formed a Steering Group to develop recommendations for IIM-associated cancer screening. A modified Delphi Method approach using a series of online surveys was utilised for recommendation formation. Draft recommendations were sent to an international group (79 members located across 22 countries) with expertise in IIM and cancer screening. Surveys asked respondents to rate their level of agreement with each draft recommendation on a 1-9 numerical rating scale (1 = complete disagreement, 9 = complete agreement). The median vote rating for each draft recommendation was calculated and defined as disagreement (median 1-3), uncertainty (median 4-6), and consensus (median 7-9). Recommendations were assigned a strength of recommendation (strong, conditional); "strong" recommendations are made when the benefits are deemed to clearly outweigh risks, whereas "conditional" recommendations are made when benefits are more clearly balanced. Recommendations were assigned a quality of supporting evidence: high, moderate, low, or very low, according to Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) methodology.
Results: A total of 18 final recommendations were generated (Table 1). Regarding strength of recommendation, 13 recommendations were strong and five were conditional. Quality of supporting evidence was moderate for 8 recommendations, low for four, and very low for three; three further recommendations had no corresponding evidence base and were formed via expert consensus only.
Firstly, recommendations allow a patient's individual IIM-associated cancer risk to be stratified into low, intermediate, and high risk according to subtype, autoantibody status, and clinical features. Secondly, recommendations outline a "basic" screening panel (including chest X-ray radiography, basic laboratory blood tests) and an "enhanced" screening panel (including computed tomography, tumour markers). Thirdly, recommendations advise on the timing and frequency of screening via basic and enhanced panels, according to low/intermediate/high risk status (Figure 1). Recommendations also advise consideration of upper/lower gastro-intestinal endoscopy, naso-endoscopy, and 18F-FDG PET/CT scanning in specific populations.
Conclusion: The 2022 IIM-associated cancer screening guideline provides, for the first time, recommendations addressing individual patient risk stratification, cancer screening modalities, and screening frequency. Implementation of the recommendations aim to facilitate earlier IIM-associated cancer detection, especially in those with high risk, thus potentially improving outcomes, including survival.
.jpg) Table 1 - Summary of all recommendations for idiopathic inflammatory myopathy-associated cancer screening
Table 1 - Summary of all recommendations for idiopathic inflammatory myopathy-associated cancer screening
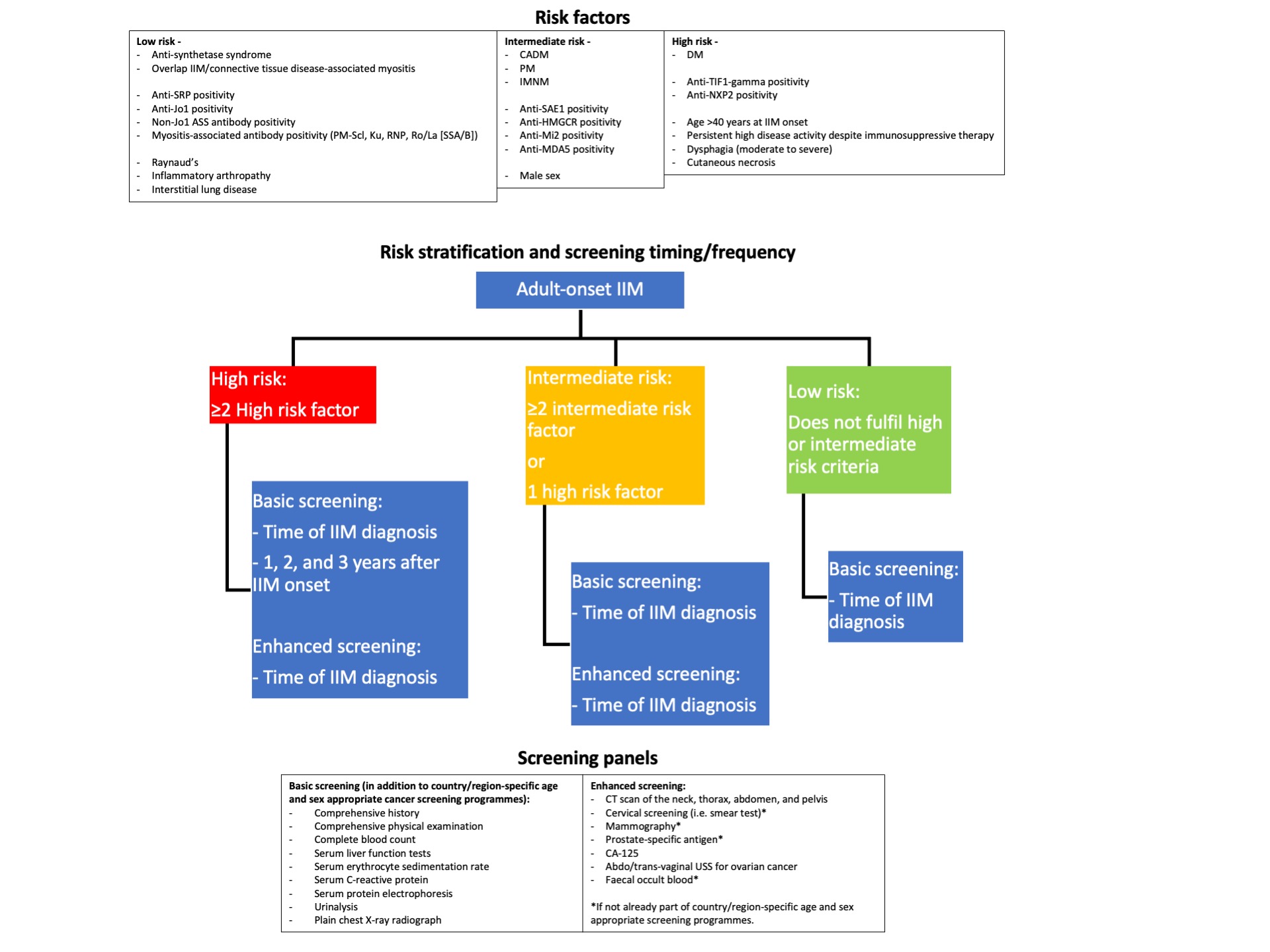 Figure 1 – Flowchart of idiopathic inflammatory myopathy-associated cancer risk stratification and timing/frequency of screening
Figure 1 – Flowchart of idiopathic inflammatory myopathy-associated cancer risk stratification and timing/frequency of screening
Disclosures: A. Oldroyd, None; J. Callen, None; H. Chinoy, Eli Lilly, UCB; L. Chung, None; D. Fiorentino, None; P. Gordon, Eli Lilly, UCB, Corbus, Galapagos; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos; N. McHugh, None; A. O’Callaghan, None; J. Schmidt, None; S. Tansley, None; R. Vleugels, None; V. Werth, GlaxoSmithKline, CLASI; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech.
Background/Purpose: Adult-onset idiopathic inflammatory myopathy (IIM) is associated with increased cancer risk (lung, ovarian, colorectal, lymphoma, breast, and naso-pharyngeal among the most common) within the three years prior to or following IIM onset. Evidence and consensus-based recommendations for IIM-associated cancer screening could potentially improve outcomes.
Methods: The International Myositis Assessment and Clinical Studies Group (IMACS) formed a Steering Group to develop recommendations for IIM-associated cancer screening. A modified Delphi Method approach using a series of online surveys was utilised for recommendation formation. Draft recommendations were sent to an international group (79 members located across 22 countries) with expertise in IIM and cancer screening. Surveys asked respondents to rate their level of agreement with each draft recommendation on a 1-9 numerical rating scale (1 = complete disagreement, 9 = complete agreement). The median vote rating for each draft recommendation was calculated and defined as disagreement (median 1-3), uncertainty (median 4-6), and consensus (median 7-9). Recommendations were assigned a strength of recommendation (strong, conditional); "strong" recommendations are made when the benefits are deemed to clearly outweigh risks, whereas "conditional" recommendations are made when benefits are more clearly balanced. Recommendations were assigned a quality of supporting evidence: high, moderate, low, or very low, according to Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) methodology.
Results: A total of 18 final recommendations were generated (Table 1). Regarding strength of recommendation, 13 recommendations were strong and five were conditional. Quality of supporting evidence was moderate for 8 recommendations, low for four, and very low for three; three further recommendations had no corresponding evidence base and were formed via expert consensus only.
Firstly, recommendations allow a patient's individual IIM-associated cancer risk to be stratified into low, intermediate, and high risk according to subtype, autoantibody status, and clinical features. Secondly, recommendations outline a "basic" screening panel (including chest X-ray radiography, basic laboratory blood tests) and an "enhanced" screening panel (including computed tomography, tumour markers). Thirdly, recommendations advise on the timing and frequency of screening via basic and enhanced panels, according to low/intermediate/high risk status (Figure 1). Recommendations also advise consideration of upper/lower gastro-intestinal endoscopy, naso-endoscopy, and 18F-FDG PET/CT scanning in specific populations.
Conclusion: The 2022 IIM-associated cancer screening guideline provides, for the first time, recommendations addressing individual patient risk stratification, cancer screening modalities, and screening frequency. Implementation of the recommendations aim to facilitate earlier IIM-associated cancer detection, especially in those with high risk, thus potentially improving outcomes, including survival.
.jpg) Table 1 - Summary of all recommendations for idiopathic inflammatory myopathy-associated cancer screening
Table 1 - Summary of all recommendations for idiopathic inflammatory myopathy-associated cancer screening  Figure 1 – Flowchart of idiopathic inflammatory myopathy-associated cancer risk stratification and timing/frequency of screening
Figure 1 – Flowchart of idiopathic inflammatory myopathy-associated cancer risk stratification and timing/frequency of screeningDisclosures: A. Oldroyd, None; J. Callen, None; H. Chinoy, Eli Lilly, UCB; L. Chung, None; D. Fiorentino, None; P. Gordon, Eli Lilly, UCB, Corbus, Galapagos; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos; N. McHugh, None; A. O’Callaghan, None; J. Schmidt, None; S. Tansley, None; R. Vleugels, None; V. Werth, GlaxoSmithKline, CLASI; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech.

